| Pages:
1
2 |
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Pigments in the art world, for love of brightly colored compounds
Sometime last year I got the bright idea that I wanted to start oil painting. Not knowing much about the art I went out and spent some money and
bought whatever colors appeared good to me online. I swear, my interests were pure and I never intended to bring chemistry into this but well...
Organic pigments are cheap, but they are not the end all. I've been learning about all the different pigments and I've been having a ball with the
obscure and toxic, the colorful and the expensive. I love the colors, and I love that mercuric sulfide gives some of the best reds and you can buy it
still just for oil painting. Cadmium yellows and reds are gorgeous and ultramarine... ahhh....
The colors are magic, almost like they should not exist in this world. The wiki article on pigment has this to say:
| Quote: | | Pure pigments reflect light in a very specific way that cannot be precisely duplicated by the discrete light emitters in a computer display. However,
by making careful measurements of pigments, close approximations can be made. |
That's and understatement. But it's not just the beauty, it's the fact that most of these are pure pigments. Prussian blue, manganese violet, one of
the things that always attracted me to inorganic chemistry was the colors and painting with pure pigments in that way is a lot like playing with your
food.
I am not a good artist, I'm terrible at painting. But that's not the point. It's beautiful, and it's chemistry.
So, does anyone else here paint? Anyone have a favorite pigment? It reminds me of when I found out about the variety of chemicals available through
ceramic stores except these are so much more beautiful though less useful for home chemistry.
[Edited on 4/8/2014 by BromicAcid]
|
|
|
wallschem
Harmless

Posts: 6
Registered: 17-11-2010
Member Is Offline
Mood: No Mood
|
|
Hi BromicAcid, I paint and I used chemistry every day and I make my living of it, you can check some of my work in my web site www.manosydedos.com I make most of my pigments just for the fun of it.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
I checked, wallschem ─ and to say I'm impressed hardly goes far enough!
There's some sense of irony, too . . . ?
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
I don't paint and actually tend to go the opposite route… using paint pigments, such as cadmium yellow, as makeshift reagents in small quantities!
I do agree though, that they produce quite beautiful and incomparable colors. I inherited the set of paints that I have from my grandmother after she
passed. She was a great artist, and while I do not have the talent, I figured that it would be fitting for me to use the paints in a different way,
for chemistry, rather than just let them sit around collecting dust.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I painted a lot back in high school actually, but have since abandoned it largely for drawing and sculpting, which produce results faster. I'm
actually in the process of producing my own pigments now using chemistry so that I can make my own paints and start again. I'm looking to make
manganese violet soon, if possible. It seems that making my own red pigment will be very problematic...
[Edited on 2-20-2015 by Amos]
|
|
|
SimpleChemist-238
Hazard to Others
  
Posts: 147
Registered: 28-9-2014
Member Is Offline
Mood: Chlorine Trifloride Flame Thrower
|
|
Any pictures?
We are chemists , we bring light to the darkness. Knowledge to ignorant, excitement to the depressed and unknowing. we bring crops to broken fields
and water to the desert. Where there is fear we bring curiosity.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Pictures? Of my paintings? I could muster a few up but they're really no where close to how I would be now.
|
|
|
argyrium
Hazard to Others
  
Posts: 123
Registered: 3-2-2008
Location: Pacific
Member Is Offline
Mood: No Mood
|
|
I use a lot of powdered pigments in my conservation work.
A good source is http://www.sinopia.com/
Great range of colors. Enjoy.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I hope you meant paintings and not pigments, SimpleChemist-238. I'm barely past ground zero on the latter.
If anyone likes anime, I draw too
   
[Edited on 2-21-2015 by Amos]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
My interest would likely be more in fibers and dying rather than painting, but I've recently been collecting onion skins and the stalks of beets with the goal of pigment extractions 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
SimpleChemist-238
Hazard to Others
  
Posts: 147
Registered: 28-9-2014
Member Is Offline
Mood: Chlorine Trifloride Flame Thrower
|
|
I like the tree!
We are chemists , we bring light to the darkness. Knowledge to ignorant, excitement to the depressed and unknowing. we bring crops to broken fields
and water to the desert. Where there is fear we bring curiosity.
|
|
|
eleusis-borodin
Harmless

Posts: 10
Registered: 20-2-2015
Member Is Offline
Mood: No Mood
|
|
This may not be the kind of "paint" you were seeking by in my study of tryptamine compounds ive found that harmala alkaloids extracted from peganum
harmala seeds glow bright white under UV light, and may be a novel source of UV reactive paint or dye.
Ive seen people use harmala alkaloid extracts as body paint or as invisible (or light yellow) but UV reactive paint in art work.
Ive heard people speculate that a UV reactive dye, which could be used to dye clothing, could be made from harmala alkaloids derived from peganum
harmala seeds.
Peganum harmala seeds are available in middle eastern markets under the names "esphand" or "harmal" where the seeds are traditionally thrown on hot
stones as an incense.
The seeds function as an mono-amine oxidase inhibitor and mild intoxicant when ingested, but most still feel this should not pose any problems when
processing the seeds into dye or paint, or when using the seed derived dyes or paints.
I hope some of this information could be useful to you, I'm an artist, and have an interest in botany, and organic chemistry, so I found this piece of
information to be novel and entertaining if nothing else, any way I hope it could help in your artistic ventures.
-E. Borodin
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I did run across an interesting pigment today (chemically):
Aureolin Cobalt Yellow
PY40—Cobalt Yellow
Chemical Name
cobalt potassium nitrite
K<sub>3</sub>[Co(NO<sub>2</sub> <sub>6</sub>]
• H<sub>2</sub>O <sub>6</sub>]
• H<sub>2</sub>O
Although in the spirit of the thread it's not the prettiest pigment in the world, a bit of a muddy yellow and expensive but interesting that it's
using nitrite as a ligand.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
http://www.danielsmith.com/ItemImages/Large/P8499B.jpg
Looks pretty enough to me! It's sad that I'm working on pigments of all things and don't even have a cobalt compound on hand.
|
|
|
ni3rtap
Harmless

Posts: 9
Registered: 21-9-2013
Member Is Offline
Mood: No Mood
|
|
I oil paint. Though about 60% of making it look good is getting the light/shadow.
I also work at Sherwin Williams doing QC/fixing batches in a plant. I'm not all that experienced, but feel free to ask me questions.
[Edited on 2-3-2015 by ni3rtap]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
I'm fond of bismuth vanadate myself. Don't paint though.
[Edited on 3-5-2015 by Etaoin Shrdlu]
|
|
|
samaterials
Harmless

Posts: 3
Registered: 9-3-2015
Member Is Offline
Mood: No Mood
|
|
Inorganic pigments are cheaper. They are usually metal compounds and mainly oxides. E.g. http://www.samaterials.com/bismuth/1113-bismuth-iii-oxide-po...
http://www.samaterials.com/holmium/1084-holmium-oxide.html; http://www.samaterials.com/antimony/1131-antimony-trisulfide...
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Cheaper than organic pigments or cheaper than buying the prepared paint? Inorganic pigment containing paints are much more expensive than their
organic counterparts in any instance I can think of. However, organic pigments can be very concentrated so the paints are cut with fillers (in some
instances) so I could see some organic pigments being more expensive on a w/w basis.
I present to you Aldrich. Cadmium sulfide, cadmium selenide, titanium dioxide, and zinc oxide.

|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
A study that I did on Hellfire by Pete Venters for practice (from Magic the Gathering).
Lots of cadmium, iron, zinc, and titanium in this one.

|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Amazing art !
Someone (artistic) U2U CHRIS25.
He'd love this.
|
|
|
samaterials
Harmless

Posts: 3
Registered: 9-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by BromicAcid  | Cheaper than organic pigments or cheaper than buying the prepared paint? Inorganic pigment containing paints are much more expensive than their
organic counterparts in any instance I can think of. However, organic pigments can be very concentrated so the paints are cut with fillers (in some
instances) so I could see some organic pigments being more expensive on a w/w basis.
I present to you Aldrich. Cadmium sulfide, cadmium selenide, titanium dioxide, and zinc oxide.
|
See the differences between Organic and Inorganic pigments below:
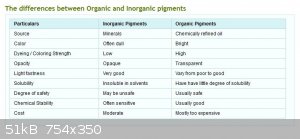
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Indeed, but I thought you were trying to make a point about price. Cost on the chart is listed as moderate for inorganic pigments and 'mostly too
expensive' for organic pigments. But my point still stands, organic pigments can be expensive on a weight vs weight ratio but they are much more
concentrated, a little goes a long way. For example, compare the Utrecht line of artists oil paints:
Cadmium orange pure - 37 mL $18.66
Cadmium orange hue - 37 mL $8.66
Cadmium red medium - 37 mL $22.65
Cadmium red hue - 37 mL $8.66
Cadmium yellow lemon pure - 37 mL $22.65
Cadmium yellow hue -37 mL $6.32
Cerulean blue pure - 37 mL $22.65
Cerulean blue hue - 37 mL $8.66
Cobalt blue - 37 mL $22.65
Cobalt blue hue - 37 mL $6.32
Where hues are mainly organic pigments. Also, if you pick up a tube of cadmium red and a tube of cadmium red hue the hue is much lighter, it's weird
at first but the cadmium is just so dense and they go by volume. So it would take even more paint to equal the same weight, but it's all about the
volume and the coverage. I use the inorganic pigments because they have better covering ability.
So, what are you getting at? I can see some scenarios where you're right but most of the time inorganic pigments just cost more in actual use (except
for white).
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Those are specialty inorganic pigments, which I think almost only the art world uses, hence price is artificially elevated. Oxides, etcetera, should
be much, much less expensive. They cost less than average quality dirt.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Yes, chemicals of a specific purity for special applications often fetch higher prices. However, I wouldn't compare the price to dirt*, even on
Alibaba a kilo of cadmium sulfide (not even pigment grade) can set you back $500. Interestingly enough I have found it at half that cost from Kremer Pigments in pigment grade. I've found cinnabar even cheaper which I found strange considering the price of mercury itself (and the
toxicity) which makes me wonder if it was cut with something.
*Cost of cubic yard of screened soil in my area $9.70 USD, approx weight cubic yard 2200 lbs (998 kg) or approx $0.01 / kg
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Yeah, cadmium sulfide is expensive. It's a specialty pigment. Nothing to do with purity, it has to do with the fact that it's only used in the art
world. Pigments that get used to color polymers, coatings, are the big movers and the cost goes down.
Go compare the cost of yellow oxide to potting soil now. 
EDIT: According to Wikipedia cadmium sulfide has some use still in high-temperature coatings, but this is still a specialty use. Cadmium pigments all
but went the way of the dodo when non-toxic organic replacements were produced.
[Edited on 3-17-2015 by Etaoin Shrdlu]
|
|
|
| Pages:
1
2 |