Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
N,N-dimethylaniline
As requested, here is the first synthesis using my homemade autoclave.
Preparation of N,N-dimetylaniline
This synthesis followed the procedure in Fundamental Processes of Dye Chemistry by Fierz-David and Blangey, 1949, p.133. This can be found in
the SM library.
Special Equipment
This synthesis requires the use of an autoclave capable of 60atm. I used my homemade autoclave, construction of which is documented in the
Prepublication section of this forum.
Reagents
Methanol was Drygas gasoline de-icer. It tested negative for ethanol and acetone in an iodoform test. Sulfuric acid (96%) was Rooto drain cleaner.
The aniline, which was amber, was from Daigger. All reagents were used “as received.”
Procedure
Due to the highly poisonous nature of aniline and N,N-dimethylaniline, all procedures were conducted in my hood using gloves.
A. 1st Heating Period (6hrs at 215C)
133mL of methanol and 91mL of aniline were mixed in a 400mL beaker using a magnetic stirrer. One mL (of 5mL) of sulfuric acid was added to the
stirring solution. This resulted in a small but violent reaction. The rest of the acid was added by the drop. The solution turned an opaque creme
color, presumably due to the formation of aniline acid sulfate. Resulting pH was 5.
The autoclave had been sealed shut, except for the fill port, using a Garlock Graph-Lock 3123 gasket. This is a 1/16” gasket made of graphite. It
was then backfilled with argon, which was probably unnecessary. The reactants were introduced at the bottom of the autoclave through the fill port
using a ¼” OD x 0.170” ID Tygon tube attached to a glass funnel. The fill port plug was then installed using Ace TFE thread sealant.
The heaters were turned on. The silicone oil bath was set at 225C. The aux heater was set at 30% (330w). Forty-five minutes later the pressure (P)
= 40 psig. At 2 hrs heating P= 460psig. At 2.5 hrs heating the thermowell temperature (Tw) = 220C. The indicated pressure was very close to that of
the procedure, ie, 30atm. At 8 hrs heating the heaters were turned off and the autoclave allowed to cool overnight.
B. 2nd Heating Period (5hrs at 170C)
The next day the fill port was opened and a 5mm glass rod was inserted to the bottom of the autoclave through the fill port. This assured that this
passage was open, and provided a minimal amount of vertical stirring. Then 18.8mL of 30% NaOH was added to the autoclave via a small screw-on funnel
made of pipe fittings.
The oil bath was set at 195C. Aux heater was set at 20% (192w). After 1 hr of heating P=55 psig and Tw = 120C. After 2.75 hr heating P = 200psig, Tw
= 176C. After 5 hours of heating the heaters were turned off, the insulation removed, and the autoclave allowed to cool overnight.
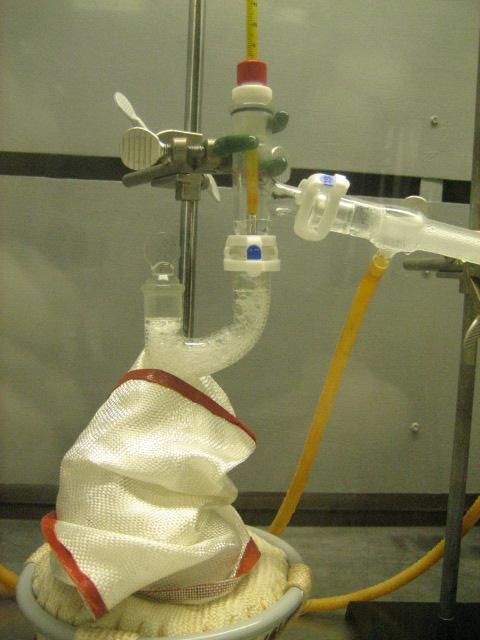
C. Steam Distillation
Set-up for steam distillation using a 1000mL 3-neck RBF as pot. Both live steam and direct heating would be used simultaneously.
Opened up the autoclave. The product was a clear liquid with about 4mL of black particles which settled upon standing. This was assumed to be
carbon. The solution pH was 11. The product was transferred to the pot using a turkey baster. The small amount of carbon sludge and water was
discarded. There was some noticeable vapor pressure in the pot, most likely due to unreacted methanol.
The steam distillation proceeded without incident. The product was placed in a separatory funnel. NaCl was added to saturate the water layer and the
organic layer was separated. It weighed 104.4g and had a napthalenic smell. I did my best not to smell it due to its highly poisonous nature.
D. Simple Distillation
I then set up for simple distillation using a 250mL RBF as pot. The procedure called for use of a small bulb column, which I did not have. Expecting
that the forerun would be off-spec a 25mL RBF was used as receiver. Expected boiling point would be 192C so ptfe joint clamps and an insulating
blanket were used. Distillation quickly proved problematic due to foam generation causing a carryover surge of pot contents into the condenser. I
then shutdown and added a Claisen head to lengthen the distillation path and impinge the foam. By careful manipution of the insulating blanket to
control the temperature of the Claisen head I managed to just keep the foam out of the condenser.
The distillate came over at a rock steady 192C and therefore was considered sufficiently pure. The net product weighed 91.4g. I probably lost about
10g due to the carryover during the foam surge. This could have been recovered but I chose not to.
Yield
The procedure expected yield was 117g. My net was 91.4g. This is a 75.5% yield based on the amount of aniline charged.
Cleanup
The small amount of sludge was not resinous, ie, not sticky and was easily removed from the autoclave. The autoclave was then sprayed down with WD-40
for storage.
Conclusions
I like this procedure as it is relatively straightforward in terms of chemistry and workup. Also it delivers the product in good yield. The long
heating times are a downside, however.
I am pleased with this performance of my autoclave. This was its first use.
[Edited on 14-10-2010 by Magpie]
[Edited on 15-10-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Dimethyl Sulfate, In-situ?
[Edited on 14-10-2010 by zed]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
BTW alternate route:
http://www.sciencemadness.org/talk/viewthread.php?tid=7512#p...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Wow, downloaded 1159 times. I wonder if everyone will be building autoclaves now?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Pffft. 1686:
http://www.sciencemadness.org/talk/viewthread.php?tid=8375#p...
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Nice work. Can you post more pictures of the autoclave? In the picture you posted I can only see the flange.
What is the max pressure rating of that flange? class 900?
[Edited on 10-15-2010 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by smuv  | Nice work. Can you post more pictures of the autoclave? In the picture you posted I can only see the flange.
What is the max pressure rating of that flange? class 900?
[Edited on 10-15-2010 by smuv] |
Thank you. See "Homemade Autoclave" in Prepublication for more pictures of the autoclave. The flanges are class 300.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I am planning to repeat this reaction ,but on para-aminophenol(from paracetamol) instead as I don't have aniline lying around 
so here is my question: Is there a chance of ether formation between the phenolic OH and the methanol ?
http://www.masterorganicchemistry.com/2014/11/14/ether-synth...
http://en.wikipedia.org/wiki/Ether#Dehydration_of_alcohols
[Edited on 26-12-2014 by CuReUS]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by CuReUS  | I am planning to repeat this reaction ,but on para-aminophenol(from paracetamol) instead as I don't have aniline lying around 
so here is my question: Is there a chance of ether formation between the phenolic OH and the methanol ? |
You do realize that the reaction only works in an autoclave at 60atm of pressure? Having this beast made seems to me, an enormous hurdle compared to
having aniline.
Anyway, with regards to p-aminophenol, I would be almost certain that alkylation of the phenol would occur (in situ formation of methylsulfuric acid
and/or dimethylsulfate being the likely mechanism, IIRC), and hydrolysis/alkylation to p-dimethoxybenzene (via a quinoid intermediate) may be a
competing reaction.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
actually I am trying to synthesize chloroxylenol ,so i came up with this route
1.p-aminophenol to p-(n,n-dimethylamino) phenol
2.hoffman martius rearrangement to 3,5 dimethyl 4-aminophenol
http://en.wikipedia.org/wiki/Hofmann%E2%80%93Martius_rearran...
3.diazotization followed by treatment with CuCl/HCl to get chloroxylenol
http://en.wikipedia.org/wiki/Chloroxylenol
but as you are saying that O-alkylation will be inevitable,then I will have to think of another method to make chloroxylenol
also,why doesn't the martius hoffman reaction take place in the autoclave itself ?
| Quote: | | with regards to p-aminophenol,....hydrolysis/alkylation to p-dimethoxybenzene (via a quinoid intermediate) may be a competing reaction.
|
I think the quinone formation can be prevented if the pH of the solution is kept low enough 
I have done the same thing while i was making dyes using 2,4 diaminonaphthol and it worked.When the 2,4 diamino was put in a beaker with 20ml conc
HCl,the oxidation was prevented
|
|
|
Yugen
Harmless

Posts: 23
Registered: 20-12-2014
Member Is Offline
Mood: No Mood
|
|
This seems like too much work
Tertiary methyl amines can be more easily prepared by the Eschweiler–Clarke reaction. Atmospheric pressure + formic acid + formaldehyde + aniline. Might want to reconsider the pressure vessel if I am
not too late. In the end a pressure vessel is never a useless piece of equipment if you already committed. 
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
yugen,S C wack has already suggested using esch-clarke earlier in the thread
but your suggestion has given me an idea about making my compound -chloroxylenol
starting off with p-aminophenol,do esch-clarke to get p-(n,n-dimethylamino)phenol
then acetylate the OH
then do hoffman martius rearrangement to get 4-acetoxy-2,6-dimethylaniline
diazotise,replace with Cl and remove the protecting group to get 4-chloro-2,6-dimethylphenol
but I have a few questions
1.will the OH ester survive the hoffman martius rearrangement conditions(heating to 300'C in presence of HCl)
http://pubs.rsc.org/en/Content/ArticleLanding/1920/CT/ct9201...
2.is there a chance of fries rearrangement? does it always require a lewis catalyst ?
http://en.wikipedia.org/wiki/Fries_rearrangement
3.are there any other easily available protecting groups for OH
other than esters and ethers(ethers also undergo hoffman martius rearrangement)
I came across another way to make n,n-dimethylaniline
http://pubs.rsc.org/en/content/articlelanding/2009/nj/b90565...
but I have a question,can raney nickel remove OH from phenol
I have found references that raney removes phenolic OH only if ether is made first
so can it not remove just OH?
|
|
|
Yugen
Harmless

Posts: 23
Registered: 20-12-2014
Member Is Offline
Mood: No Mood
|
|
@CuReUS
1. Doubtful, esters can be hydrolyzed (maybe under anhydrous conditions it'd work...)
2. Fries Rearrangment's reaction mechanism shows a lot of electron juggling between the carbonyl group and the catalyst (there's a nice pic on that
wiki link you posted).... So probably not a problem here without a catalyst.
3. Why protect at all? Phenols are tough as hell to remove (I can't think of anything tougher actually).
4. Just buy and distill Dettol (a common antiseptic)! Get your chemical without all this hassle!
If that isn't challenging enough for you then just chlorinate xylenol (the directing groups on the ring should give almost entirely the para product
you're after).
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
but I have found a reference for thermal hoffman martius rearrangement so that should not be a problem
| Quote: | | 3. Why protect at all? Phenols are tough as hell to remove (I can't think of anything tougher actually). |
because in the reaction mechanism of Ho-Ma rearrangement ,methyl carbocation is produced,which might alkylate the OH instead of attacking the ring.I
am not scared that It will get removed 
| Quote: | | 4. Just buy and distill Dettol (a common antiseptic)! Get your chemical without all this hassle! |
I plan to synthesize it, I dont have any need for it
| Quote: | | If that isn't challenging enough for you then just chlorinate xylenol (the directing groups on the ring should give almost entirely the para product
you're after) |
I am sure many members,including me have wet dreams everynight about which chemicals we would like to work with
AFAIK,humans don't shit out xylenol,nor does it grow on trees
please tell a cheap and good source of xylenol and I will reconsider my opinion
also which chlorinating agent should be used ?
just passing chlorine through xylenol in the presence of HCl?
[Edited on 26-1-2015 by CuReUS]
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by Yugen  | Tertiary methyl amines can be more easily prepared by the Eschweiler–Clarke reaction. Atmospheric pressure + formic acid + formaldehyde + aniline. Might want to reconsider the pressure vessel if I am
not too late. In the end a pressure vessel is never a useless piece of equipment if you already committed.  |
I just tried this and got a large quantity of disgusting yellow stringy sticky goo. I assume it is some kind of polymer. Apparently, aniline
formaldehyde resins are a thing....
|
|
|