| Pages:
1
2
3 |
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by blargish  | Quote: Originally posted by nlegaux  | I got some 57% Sodium Dichloroisocyanurate the other day. I guess I will recrystallize it and try making the Copper Complex  |
Here is a cool document with some information about the complex, and the procedure for making it. Apparently, the complex is of the form,
Na2[Cu(C3N3O3Cl2)]
|
zts16 has a lovely picture here. http://www.sciencemadness.org/talk/viewthread.php?tid=45845&...
I feel somewhat inspired. The colour is really pretty. I don't have any sodium dichloroisocyanurate though. I do have some TCCA and if I am not
mistaken I can make some by reacting with NaOH.
Thanks for the link although I haven't read much of it yet. There are loads of possibilities. I will also substitute NaOH for KOH and see what the
potassium variant looks like. And I can substitute Cu for Pb, Ni, Co and possibly Mn. This could be a fun little project.
By the way, in this post, Nicodem reports a molecular formula different from what you have stated above. Apparently there are 4 dichloroisocyanurate ligands.
Quote: Originally posted by Nicodem  | The reaction of CuSO4 and sodium dichloroisocyanurate in water is described in US3055889 and is claimed to give Na2[CuL4] where
L is the dichloroisocyanurate ligand: (C3N3O3Cl2)-. Described are also the preparation of
analogous Ca, K, Ba and Li tetrakis(dichloroisocyanuro)cuprates (or whatever they are called). Analogous Cd and Ni coordination compounds are also
reported.
US3115493 describes the preparation of a lead dichloroisocyanurate and this is then used in the preparation of Pb[CuL4].
An analogous potassium nickelate dichloroisocyanuric complex is also known (DOI: 10.1016/0022-1902(64)90014-4).
[Edited on 14/3/2014 by Nicodem] |
|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
Quote: Originally posted by j_sum1  |
By the way, in this post, Nicodem reports a molecular formula different from what you have stated above. Apparently there are 4 dichloroisocyanurate ligands.
[Edited on 14/3/2014 by Nicodem][/rquote] |
Oops, my bad. There formula given in the document is actually Na2[Cu(C3N3O3Cl2)4],
you're right.
BLaRgISH
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
blargish, I mispoke in my previous post, what I think I have is copper (II) chromate, which is described in the CRC as reddish brown crystals with the
formula of CuCrO4.
nlegaux, the 70% ethanol I used for my recrystallization can be bought cheaply at most pharmacies and works well. Good luck with your isolation.
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Trying to recrystallize Ibuprofen with IPA failed... No crystals formed when water was added, even when chilled overnight. I guess I will have to go
buy some Ethanol 
[Edited on 11-30-2014 by nlegaux]
|
|
|
sum
Harmless

Posts: 20
Registered: 9-1-2015
Member Is Offline
Mood: No Mood
|
|
Hi,I am new in this.I want to know how to make copper(||)acetoarsanite (Paris green).this is one of copper compound.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I like making Fehling's solution, although you kinda get a mix of compounds if you crystallize it out, it's still pretty enough to 'collect'.
Try copper(II) ferricyanide and copper(II) ferrocyanide, both different brown-like colors.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by sum  | | Hi,I am new in this.I want to know how to make copper(||)acetoarsanite (Paris green).this is one of copper compound. |
So why not look up a preparation for it? Simply google searching it seems to indicate one can just add arsenic trioxide to a copper acetate solution.
It might be more complicated, so maybe do some research.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Quote: Originally posted by Zyklon-A  | Thanks. Hydrobromic acid and copper (II) hydroxide or carbonate.
HBr (aq) was made from H2SO4 and NaBr I think - not an easy reaction to do, cause it partially oxidizes bromide to bromine.
I ought to find a better way to make such a useful acid. |
There is a better way to make CuBr2, without the acid.
NaBr + CaCl2 <--> NaCl + CaBr2
CaBr2 is about 5 times as soluble as NaCl, so it is easily purified by concentration, filtering most of the NaCl, then fractional crystallization.
CaBr2 + CuSO4 --> CuBr2 + CaSO4
Calcium sulfate is 265 times less soluble than CuBr2 (basically insoluble) and can be filtered out, leaving a solution of CuBr2.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Praxichys  | Quote: Originally posted by Zyklon-A  | Thanks. Hydrobromic acid and copper (II) hydroxide or carbonate.
HBr (aq) was made from H2SO4 and NaBr I think - not an easy reaction to do, cause it partially oxidizes bromide to bromine.
I ought to find a better way to make such a useful acid. |
There is a better way to make CuBr2, without the acid.
NaBr + CaCl2 <--> NaCl + CaBr2
CaBr2 is about 5 times as soluble as NaCl, so it is easily purified by concentration, filtering most of the NaCl, then fractional crystallization.
CaBr2 + CuSO4 --> CuBr2 + CaSO4
Calcium sulfate is 265 times less soluble than CuBr2 (basically insoluble) and can be filtered out, leaving a solution of CuBr2.
|
Yup. Of course, fractional crystallization isn't the easiest, but still.
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
Here's an idea for displaying your copper compounds. I tried to recreate the peg game that you find at Crackle Barrel with copper compounds and it
turned out pretty good.
The compounds are sealed in culture tubes and the triangle is machined acrylic. I have since made a new and improved version, but I don't have a
picture handy at the moment.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Thanks for that Praxichys, I'll be sure to use that method next time and save what little HBr(aq) I have left.
Beautiful collection Squall181! I know what most of those are, but could you list them?
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
In my experience copper ferricyanide is a yellow color.
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
The one's visible in the picture from top right are: Copper Acetate, Copper(II) Chloride, Copper(II) Oxide, Copper, Tetraaminecopper(II) sulfate. The
rest are copper nitrate, copper carbonate, copper bromide, copper(I) oxide, copper(I) chloride, copper sulfate, copper sulfide, Copper Cyanurate, and
copper aspirinate. I believe that all the ones I have.
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Squall181  | Here's an idea for displaying your copper compounds. I tried to recreate the peg game that you find at Crackle Barrel with copper compounds and it
turned out pretty good.
The compounds are sealed in culture tubes and the triangle is machined acrylic. I have since made a new and improved version, but I don't have a
picture handy at the moment.
|
This looks really, nice, I'll have to see what local prices for Plexiglas are. Here is a picture of my collection. Copper benzoate was made after this
picture was taken and hence not in it.

From left to right:
Chevreul's salt - Copper I iodide (with water) - Pyridine copper iodide - tetrachlorocopper complex - Copper oxalate - Copper II chloride - Copper
stearate - Copper carbonate - Copper acetate - Copper oleate - Copper (durr) - Copper aspirinate - Copper II sulfate - Copper nitrate -
Tetraaminecopper II sulfate - Pyridine/Copper nitrate complex - Copper phthalocyanine - Copper sulfate/cyanurate - A mislabeled vial of decomposed
copper complex (I have no clue where it came from I found it in a corner of a shelf, but I have no clue what it is.) - Copper bromide - Copper II
oxide
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Copper (en) perchlorate is fun. Woelen & myself have synthesised this from copper sulphate, ammonium perchlorate and ethylene diamine.
Attachment: Copper.mp4 (1.2MB)
This file has been downloaded 804 times
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I've been doing a lot of Iron cyanide indicator experimentation recently, it has always had a yellow brown color, with a tint of green, but I just
call it brown. It's kinda brown mustard colored for me. I've seen with coloration for others, it has to do with the concentration of the ferricyanide
and the concentration of the iron cyanide, which change crystal size.
Nezza, that does look fun!
Numos, your collection is great!
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Hey guys try copper tungstate by treating sodium tungstate and copper sulfate or nitrate solutions to obtain dense copper tungstate precipitate.
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
and try potassium bisoxalatocuprate dihydrate
[Edited on 16-1-2015 by sasan]
 
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
oh I have forgotten copper tetraiodomercurate
[Edited on 16-1-2015 by sasan]
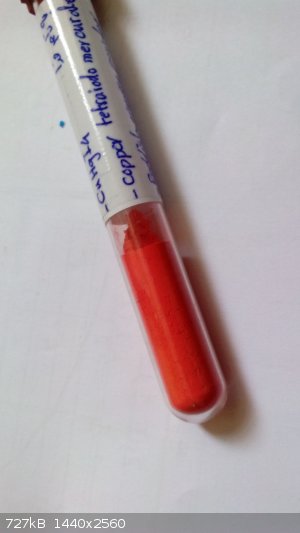
|
|
|
warteo
Harmless

Posts: 42
Registered: 30-3-2011
Location: Oz
Member Is Offline
Mood: No Mood
|
|
The mention of an orange Cu(II) complex in this paper Copper(II) Complexes With Lignin Model Compound Vanillin got me interested. The synthesis appeared easy so I gave it a go:
[Cu(C8H7O3)2(H2O)2] (2): [Cu2(O2CCH3)4(H2O)2]
(0.160 g) was dissolved at room temperature in 10.0 mL
of acidified water (one drop of glacial acetic acid). The
solution and solid vanillin (0.244 g) were slowly heated
in two separated flasks. When vanillin had melted, the
acetate solution was put over melted vanillin that turned
red and dissolved in ~5 seconds. Orange crystals of 2
were filtered off next day and dried in air for a day.
However both of my attempts following the above failed. There was obviously a reaction because the blue copper acetate solution instantly turned the
most beautiful emerald green upon addition to the molten vanillin. Upon cooling a large crop of pale green needle crystals formed which are quite
possibly compound 1.
Anyone else care to try this and see if they can achieve the orange compound? I'd really like to know where I went wrong (currently suspecting not
high enough temperature at mixing time). I'll need to make some more copper acetate before making another attempt.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by warteo  | The mention of an orange Cu(II) complex in this paper Copper(II) Complexes With Lignin Model Compound Vanillin got me interested. The synthesis appeared easy so I gave it a go:
[Cu(C8H7O3)2(H2O)2] (2): [Cu2(O2CCH3)4(H2O)2]
(0.160 g) was dissolved at room temperature in 10.0 mL
of acidified water (one drop of glacial acetic acid). The
solution and solid vanillin (0.244 g) were slowly heated
in two separated flasks. When vanillin had melted, the
acetate solution was put over melted vanillin that turned
red and dissolved in ~5 seconds. Orange crystals of 2
were filtered off next day and dried in air for a day.
However both of my attempts following the above failed. There was obviously a reaction because the blue copper acetate solution instantly turned the
most beautiful emerald green upon addition to the molten vanillin. Upon cooling a large crop of pale green needle crystals formed which are quite
possibly compound 1.
Anyone else care to try this and see if they can achieve the orange compound? I'd really like to know where I went wrong (currently suspecting not
high enough temperature at mixing time). I'll need to make some more copper acetate before making another attempt. |
That's cool- I didn't think vanillinate would make that good of a ligand. I may have to try that. It strikes me as odd, though, that a change in the
hydration (without a change in the coordination) would give such a strong change in colour. I suspect that their formulation of compound 2 was
incorrect- I wonder if they ever did get a crystal structure?
ETA: They did get a crystal structure, and it is the cis isomer. Z. Naturforsch. 60b, 1273 – 1277 (2005);
Has anyone ever made KCuCl3? It's supposed to form ruby-red crystals, but I've never managed to get it to work.
[Edited on 16-1-2015 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
I've never done it personally, but I've come across a prep for it online (the source is attached).
Preparation of Potassium Trichlorocuprate (II)
Dissolve 1.0g of Potassium Chloride (KCl) in 3.7 mL of distilled Water. In a fume hood,
dissolve 5.5g of Cupric Chloride Dihydrate (CuCl2•2H2O) in 50 mL of concentrated HCl in a
250 mL Erlenmeyer flask. Stir the mixture until it is dissolved; this may take several minutes.
Do not heat the mixture as this will drive off the HCl. Essentially dropwise, pour the KCl
solution into the CuCl2 solution slowly and with rapid stirring. Place the Erlenmeyer in an Ice
bath for half an hour. Vacuum filter the red, needle-like crystals in a medium porosity sintered
glass crucible. Spread the damp precipitate onto a watch glass and dry them in an oven that is
not any hotter than 85oC. Once dry, weigh the product and then store the sample in a 3 dram vial
flushed with Nitrogen and sealed with Parafilm wax.
Is this the procedure that you attempted?
[Edited on 16-1-2015 by blargish]
Attachment: Two Interesting Copper Compounds.pdf (299kB)
This file has been downloaded 1686 times
BLaRgISH
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blargish  |
I've never done it personally, but I've come across a prep for it online (the source is attached).
Preparation of Potassium Trichlorocuprate (II)
Dissolve 1.0g of Potassium Chloride (KCl) in 3.7 mL of distilled Water. In a fume hood,
dissolve 5.5g of Cupric Chloride Dihydrate (CuCl2•2H2O) in 50 mL of concentrated HCl in a
250 mL Erlenmeyer flask. Stir the mixture until it is dissolved; this may take several minutes.
Do not heat the mixture as this will drive off the HCl. Essentially dropwise, pour the KCl
solution into the CuCl2 solution slowly and with rapid stirring. Place the Erlenmeyer in an Ice
bath for half an hour. Vacuum filter the red, needle-like crystals in a medium porosity sintered
glass crucible. Spread the damp precipitate onto a watch glass and dry them in an oven that is
not any hotter than 85oC. Once dry, weigh the product and then store the sample in a 3 dram vial
flushed with Nitrogen and sealed with Parafilm wax.
Is this the procedure that you attempted?
[Edited on 16-1-2015 by blargish] |
Yes, from the same source. No red needles or red precipitate of any kind.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I've tried to prepare KCuCl3 by a different method; according to Atherton Seidell's 1919 book on solubilities (p. 268), a mixed solution of
KCl and CuCl2 at elevated temperatures (above 72 C or so) will precipitate this double salt as it evaporates, if the compounds are present
in roughly equimolar amounts. Had difficulties with the temperature control and set the solution aside, though.
At lower temperatures it appears that a different, blue-green double salt is precipitated from aqueous solution:
CuCl2.2KCl.2H2O. I suppose that the use of concentrated HCl in the prep you mention changes things so that this compound isn't
the result.
The less you bet, the more you lose when you win.
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Wow, Those complexes look really nice! If I can get some of the reagents together, I will have to attempt to prepare them.
Where do you buy your Sodium Tungstate? All I have been able to find is a product on amazon (50 grams or so for $100). Since this is the only
synthesis for it I would have planned, I am not ready to drop that kind of money on it.
|
|
|
| Pages:
1
2
3 |