Scratch-
Hazard to Self
 
Posts: 60
Registered: 22-2-2005
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Sulfamic acid
I have read some of the other posts here on sulfamic acid, I was wondering if I could make H2SO4 in a solution so I dont run the risk of blowing up my
good glassware. When I set up some better apperatus I was going to use the S + KNO3 => SO3 + KN reaction.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
H<sub>2</sub>SO<sub>4</sub> is sulfuric acid
Sulfamic acid = HSO<sub>3</sub>NH<sub>2</sub>
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
In aqueous solution, it would probably exist as a zwitterion, with both negative and positive charges, like an organic amino-acid, (-)SO3-NH3(+).
|
|
|
Scratch-
Hazard to Self
 
Posts: 60
Registered: 22-2-2005
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
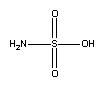
Atually, I looked up its structure (See picture below). Its MSDS says:
"Decomposes with heat (209°C) to release sulfur dioxide (SO2), sulfur trioxide (SO3), nitrogen (N2), water (H2O), and ammonia gas (NH3)."
Also:
"Hazardous reaction in aqueous solution may occur with chlorine, hypochlorous acid, hypochlorites, cyanides, nitric acid, or sulfides. An
explosion occurred when chlorine was being passed at room temperature into a reaction mixture which included sulfamic acid and water. It is suspected
that nitrogen trichloride, a very sensitive explosive, was formed. Fuming nitric acid combined with sulfamic acid causes violent releases of nitrous
oxide."
So wow, I'm glad I know that now.
I was using it to dissolve pennies, which I was trying to get the zinc out of. I was then going to use my pathetic Drano crystals NaOH to precipitate
out fairly pure zinc powder (I would just need to remove the soap and aluminum from the NaOH, which is in clumps). Reverend Necroticus Rex tells me
that zinc sulfamate (Is that name correct?) is a powerful reducing agent.
Do any of you think I could get SO3 (Safely) out of this and thus anhydrous sulfuric acid?
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
I didn't actually mean that the sulfamate salt itself was a reducing agent, I'm not too sure about that.
I read that in a few MSDS sheets, and I think they might mean, that it erodes the surface layer of oxides on the metal, in a similar way to the action
of HgCl on aluminium foil, as used in for...certain types of organic chemistry
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|