| Pages:
1
..
23
24
25
26
27
..
38 |
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I can't help but agree with MrHomeScientist! My pants and labcoat are well-worn but entirely intact.
Could it be because I work in a steady stream of air over the bench? I also generally pour stuff down a stirring rod to prevent splashing, and put
watch glasses over everything to keep dust out of solutions.
Keep a junk towel in the lab to avoid the habit of drying your hands or glassware on your pants/labcoat.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
When pouring con sulfuric acid I wear gloves, goggles, and a plastic apron. This acid is very dense and spatters readily - likely into droplets not
easily seen. The apron has stopped the generation of "moth holes" at the waistline of my shirts.
I agree that doing transfers in the hood with the fan on prevents breathing dusts and small droplets. This is especially important when transferring
particulate NaOH.
I try to practice good housekeeping and hygiene. I'm always surprised to see how much my disposable nitrile gloves are discolored after a day in the
lab.
[Edited on 26-8-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by HgDinis25  | | MrHomeScientist, when handling concentrated sulfuric acid there's absolutly no chance you're going to avoid holes in cotton made clothes. It just
happens. Back in the day I remember PIPPTETING a sample of Sulfuric Acid (using glass pippetes) and finding my t-shirt with those tiny holes, in the
following day. Coincidence or not, it has happened to me more than once. |
Well again I wouldn't say it's completely unavoidable. I've avoided it so far, and I've been in the hobby 5 years handling conc. sulfuric for probably
3 of those years. Pouring it, pipetting it (in glass and plastic droppers), reacting it, the works. Never have I seen holes in the everyday clothes I
wear. Maybe I'm just lucky, but I think with good practice you can prevent it. Just be careful is all.
[Edited on 8-26-2014 by MrHomeScientist]
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
Just out of curiosity, the clothes on you on lab duty are mostly cotton or they contain other polymers?
I'm thinking that, perhaps, cotton clothes are less resistant towards dehydration from Sulfuric Acid...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Laziness and Stupidity can be very Painful.
Ethanol Distillation again, and i was too lazy to wash out any of my favourite 250ml RBFs.
Just the 3-neck 250ml left, which i somehow don't like as much as the single neck versions.
So, distill 250ml, empty the vessel, add fresh stock, distill again. 25~30 minute cycle as usual.
Usually i don a thick rubber glove and manhandle the single neck out, empty/refill it.
Aha ! 3-neck has Openings !
I'll just syphon out the spent liquid, refill, stopper up and good to go again.
Idiot plan : I just stuck a half meter tube in there and sucked.
Very Hot liquid very quickly burnt my mouth and face.
The liquid moved very very fast.
In shock, i must have rapidly removed it from mouth, and hovered it over my right leg as it kept on syphoning.
Summer here, so Shorts. Sitting down as well.
Mucho hot liquid all over Bare right leg.
Brain re-engaged (it was definitely in chaos for a second or two) and the pipe was dropped.
Quickly grabbed the wash bottle, and squirted on leg.
DIW was about 40 C, so no good.
Grabbed 5L bottle of DIW. Splashed it all over. Same.
Ran to pool, jumped in. That helped me, but not the Watch.
The body's response to dead, cooked dermal tissue started after about 4 minutes in the pool.
That stings a bit.
I got out of the pool and noticed that the pain subsided a little with the cooling effect of the water evaporating, so rigged up a fan and got some
kitchen towel and some of the distillation coolant water (back in the lab at this time) and started wetting/blowing/evaporating.
After about 30 minutes of wetting/blowing with the fan, the most damaged areas began to look like uncooked, chicken flesh.
Blistering started after about an Hour.
Noticeable pain lasted for less than an hour
With some snipping and probing, it luckily turns out to be only 1st degree burns, so the same as a Graze really, so no real damage.
The Amazing thing was the Right Leg's behaviour after being scalded.
Naturally i carried on distilling, after rigging a Vacuum flask and some pipes to safely suck out the spent liquid, but my Right Leg refused to be
anywhere near anything hot.
I Smoke. When i lit a cigarette, the leg twitched.
Standing next to the disty rig, it was appreciably difficult to get that leg to go anywhere near the heat source.
Learning can be such fun !
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Man that sucks. So was the ethanol at it's boiling point?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I just had an ammonium sulfate solution that I was concentrating boil over onto the hot plate while I left the lab for a few seconds to look up its
solubility (as I had forgotten to look it up before.) The effect was similar to a smoke bomb, and ammonium sulfate particles are very irritating.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ethanol had gone, so ~100.
A sulphate bomb ! Good call.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
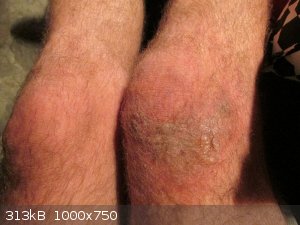
Furry blisters
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's horrid aga, so sad :/
Haven't broken anything recently.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I was making potassium iodate with potassium permanganate and went to vac filter out the MnO2 when *dink* my 11cm Buchner funnel randomly cracked I bought it new and only used it 3 times, I'm going to e-mail the seller *cough*
Scientific Equipment of Houston aka SEOH on Amazon *cough* Now I can't vac filter, and I lost half of the liquid to a brown sludge flowing on my
driveway I bought it new and only used it 3 times, I'm going to e-mail the seller *cough*
Scientific Equipment of Houston aka SEOH on Amazon *cough* Now I can't vac filter, and I lost half of the liquid to a brown sludge flowing on my
driveway
The liquid was like 70°C, the porcelain shouldn't be cracked from the thermal stress, right?
[Edited on 10-3-2014 by gdflp]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sad that i was so stupid, yes.
Jumping in the Pool was a good plan.
All healed up without scabbing, which was remarkable.
Still furry, but no blisters anymore, and no scarring. 100% ready for the next time.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
But you did not let being burned stand between you and carrying on the distillation of more wondrous Spirits.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I wasnt going to fess up but decided I would. I saw a fractionating column for sale, 1 meter long!!! quickfit joints never used before, it also came
packed with rings and a heating jacket.
the price? £130. I thought it was a bargain but was short of cash so I sold my beloved bike to a kid at school, the bike was worth around £250 but I
needed quick cash so let it go for £100.
I got dad to drive me the 40 miles to go pick up the column as i didnt want to risk posting. Traffic was awful and it took us 2 hours to get there, on
the way back I could here it move around in the boot so when dad said he was going to stop to get fuel I decided I would rearrange it in the boot to
stop it moving.
He pulled into the petrol station and I jumped out to open the boot, I grabbed the column and lifted it out.
Dad said something as I did it so I turned with it in my hand, part was still poking inside the boot and as I swung round.....................Crack
against the side of the boot! One gorgeous column in half  . .
No bike and no column!!! All those distillations I had planed  . .
The other one I dare not detail but the long and short is, I recently got the chance to do a fire drill and test the fire extinguisher! it worked
really well. The curtains (new) were ruined and the ceiling needs a coat of paint  , but all is well and it could of been worse! I wont be lazy next time and will use the oil bath instead of a bunsen , but all is well and it could of been worse! I wont be lazy next time and will use the oil bath instead of a bunsen 
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
So sorry to hear that Little_Ghost!!!!!! 
I once broke a distillation flask the day after it was given to me, and had to wait two years before I got another one. I never got to even use it!

|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
Damn Aga. Ouch. Just reading that made me hurt (and the pictures helped).
Ghost. I have a drawer with my accidents in it in the hope that I might one day find a glass blower to repair them lol. Namely a virgrex column and a
couple of condensers minus joints. Heh. It's ok to dream
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
Romain
Hazard to Self
 
Posts: 63
Registered: 23-12-2013
Member Is Offline
Mood: Crystallized
|
|
I broke my 2 250 ml beakers... Now I'm stuck with 100 ml and 600 ml beakers, nothing in between...
I was trying to concentrate H2SO4 by boiling it. I did this in the middle of my lawn, 10 meters away from everything. The sulfuric acid boiled over,
spilled on my cheap kitchen hotplate, which is now ruined, and I stupidly cooled the whole thing, including the 350°C beaker with cold water...
Arrgh!
I also broke my only ground glass erlenmeyer flask while boiling down sulfuric acid... again. I wanted to test the conc. sulfuric acid so I cooled the
flask in HOT water this time... but it still shattered!
Anyway, lesson learned, DO NOT cool hot glassware with water, don't be impatient.
|
|
|
Jylliana
Hazard to Others
  
Posts: 126
Registered: 3-10-2014
Location: The Netherlands
Member Is Offline
Mood: Bubbly ^-^
|
|
Hehe.. That little episode of stupidity happened to me more often than I can count. I don't seem to learn from my mistakes.. 
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Whilst helping a Chemistry Teacher as a lab assistant... Students were doing the typical thermal demoposition of Potassium Chlorate into oxygent.
Basically, they had this very, very, very small test tube, containing the KClO3, which was connected to a rubber hose.
So I was helping out one group of students, when I suddenly heard a loud bang across the lab.
Turns out, another group of students (Which were meant to be supervised by the Teacher) had added too much potassium chlorate into the tube. Heated
it, melted it, and then somehow managed to incline the tube enough for the molten potassium chlorate to come into contact with the rubber hosing.
Needless to say the tube shattered, sending a drop of molten potassium chlorate flying right at this girl, who just so happened to be wearing a low
cut T-Shirt. (Lab coat didn't really help)
And, of course, as is common with Students, her immediate reaction was not spraying cold water, but rather jumping back and holding her hands to the
burn (burning herself more in the process). Teacher sent her off to the bathroom with other students.
Thankfully, after hitting her, most of the molten stuff had jumped off and dripped to the floor, protecting her from serious damage.
I was just an assistant, so couldn't really do much, even less so considering the teacher was supervising that group...
But hey, could've been much worse. 
|
|
|
Dr.Bob
International Hazard
    
Posts: 2754
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Little Ghost's story reminded me of one of my friend Ed's old stories. He used to be quite the distillation expert, separating isomers of very
close BP with very elaborate distillation columns and distillation heads. Back in his days, there were glass blowers at every local university, and
most took side work for cash.
So Ed had broken some piece on a rather large vacuum jacketed distillation head which was very elaborate, and took it to the glassblower to repair,
which he did, annealed it, and Ed picked it up a few days later. He put it on some pillows in the back seat of his big old American land yacht (an
old Ford Crown Victoria or so) and was driving the 5 miles back to his small lab building. About halfway there, someone was not paying attention
(and this was way before cell phones came along) and rear ended him-just enough to cause the whole piece to slide off of the seat and drop to the
floor, making a sad "tinkling" noise, the vacuum head being imploded. Whereas before it had only had a small bit of damage, that time the head was
destroyed completely. So he had to go to small claims court to collect (also in the days before liability insurance and lawyers suing everyone) and
order and entire new head which took weeks to get. I am pretty sure that he cobbed together something that worked OK for a while, but for some tasks
he had a special head made for only one function. Some had to handle low MP compounds, so the entire head was sealed to let the dist. head be
warmed, rather than cooled. Others had multiple distillation columns linked together somehow.
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Today was one of THOSE days.
First I wasn't looking at what I was doing, and knocked my ceramic pestle off my workbench, breaking it.
Then I (for some reason) thought I could heat water in a mason jar, which shattered that.
THEN I dropped one of my best, most-used beakers when trying to clean it, which broke it.
I'm hating myself right now.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Not a significant loss.
I was trying to add an side-arm to a borosilicate test tube, without knowing much about glass blowing, and ended up breaking the test tube under the
heat.
I will be doing some more reading before attempting again.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Loptr  | Not a significant loss.
I was trying to add an side-arm to a borosilicate test tube, without knowing much about glass blowing, and ended up breaking the test tube under the
heat.
I will be doing some more reading before attempting again. |
I hate to say it, but lol. That totally sounds like something I'd do.
Also, I totally didn't know you couldn't heat things in mason jars..... why can't you?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2754
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
You can heat things in mason jars, just not quickly or with a very hot source of heat. They are made of soda lime glass which expands a lot with
heating, unlike Pyrex or other glasses designed to handle harsh heating well (low coefficient of expansion). But most glasses will break if heated
too fast or unevenly. Round pieces are stronger in general, which is one reason why round flasks are used, not square ones.
If you are trying to modify text tubes, make sure that they are the same type of glass as the tubing that you have. If you have a soda lime tube and
borosilicate glass tubing, they will be very hard to connect together, or for the opposite as well. I see more and more test tubes made of cheaper
non-borosilicate glass nowadays.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Dr.Bob  | You can heat things in mason jars, just not quickly or with a very hot source of heat. They are made of soda lime glass which expands a lot with
heating, unlike Pyrex or other glasses designed to handle harsh heating well (low coefficient of expansion). But most glasses will break if heated
too fast or unevenly. Round pieces are stronger in general, which is one reason why round flasks are used, not square ones.
If you are trying to modify text tubes, make sure that they are the same type of glass as the tubing that you have. If you have a soda lime tube and
borosilicate glass tubing, they will be very hard to connect together, or for the opposite as well. I see more and more test tubes made of cheaper
non-borosilicate glass nowadays. |
Thanks!
I don't mind the low quality test tubes, as I use test tubes a lot, so as long as I can tell what they're made of, I often buy a lot of cheap ones,
and a few of the more expensive ones.
|
|
|
| Pages:
1
..
23
24
25
26
27
..
38 |