blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Iron in Molybdenum filament supports
When I was still contemplating entering into the Rador competition with molybdenum blue I wanted to get some molybdate from light bulb filament
supports. I have ammonium orthomolybdate but that’s not very OTC. So years of hoarding about 50 kaput bulbs would come in handy.
I snipped off about 1 g of these supports (carefully avoiding the filaments or what was left of it) and first tried to digest them with KOH/KNO3
fusion. Contrary to W, I didn’t get much reaction at all. Neutralising the KOH yielded measly amounts of MoO3, with most of the supports more or
less intact, clearly attacked but not digested.
But something interesting struck me: the washed remains of these supports were magnetic vis-à-vis the nickel crucible used for the fusion. It struck
me as odd but thought no more about it.
I then tried to dissolve the remainder of the supports with 70 % HNO3 and to my surprise the reaction was vigorous and dissolution took only a few
minutes. Lots of thick NO/NO2 fumes.
Also to my surprise the solution was orange to red. Testing for iron with thiocyanate showed Fe<sup>3+</sup> to be the cause. And of
course the magnetism was explained too.
Neutralising that solution caused thick wads of Fe(OH)<sub>3</sub> to drop out.
So, who would expect significant amounts of iron in these Mo filament supports?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Very interesting. It looks like my element collection needs a new specimen!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Where did you found out that filament supports contain molybdenum? I googled about it, if there is any information about their composition, but the
only (unreferenced) claims were about them being made from an alloy of iron and nickel.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
It appears to be a pretty commonly-mentioned thing across the web, though I've not been able to find anything that links back to an actual light bulb
manufacturer. However there are several bulb diagrams that include the Mo wires:

Source: http://help.tradingdepot.co.uk/lighting/crompton-lighting/co...
This info is referenced to Crompton Lighting, an Australian lighting supplier.
Here's a book on Molybdenum that makes mention of it: http://books.google.com/books?id=nwpXX7FvtMUC&pg=PA20&am...
This specialist lamp company, USHIO, says on its faq :
"There are two kinds of bulbs used in halogen lamps: transparent and translucent quartz bulbs. Materials inside the halogen lamp include tungsten
filament, molybdenum foil, and filling gases consisting of a combination of Nitrogen (N2), Argon (AR), Krypton (Kr) and a small
amount of halogen gas made up of iodine (I), bromine (Br), chlorine (Cl), and fluorine (F). The base of the lamp is usually made of steatite or
heat-resistant metal."
A bit different than incandescent, but it does show that Mo is used in lighting. Here's a corresponding image for these halogen bulbs:
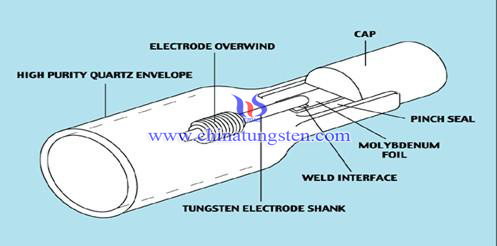
Source: http://www.molybdenum-foil.com/Molybdenum-Foil-in-UV-Lamps.h...
The above source also has a "molybdenum uses" page that cites filament support wires: http://www.molybdenum.com.cn/molybdenum-uses.html
That site has Mo in the name, so I'd be inclined to believe them!
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I suspect this
http://en.wikipedia.org/wiki/Kovar
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | Where did you found out that filament supports contain molybdenum? I googled about it, if there is any information about their composition, but the
only (unreferenced) claims were about them being made from an alloy of iron and nickel. |
I read this recently somewhere reputable but don't remember rightly where. I think it might have been Holleman's 'Inorganic Chemistry'.
Wouldn't iron/nickel be short lived at least near the filaments?
[Edited on 9-12-2014 by blogfast25]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Not if they are thick enough.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'm thinking of the tips, touching the filaments: evaporation of the Fe and of Ni.
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Some wiki stuff here
http://en.wikipedia.org/wiki/Glass-to-metal_seal
The thermal expansion suits.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I've checked it and it was indeed Holleman's 'Inorganic Chemistry' that said that W is for filaments, Mo evaporates to quickly for that but is used
for filament supports among others because it can be melted into glass easiliy.
I will test the alkaline solution for Mo later on.
[Edited on 9-12-2014 by blogfast25]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Most of the bulbs I have seen have the filament connected by folding the feed-wire over it.
in this image, you can see that the ends of the support wires are thicker.
http://mirror-uk-rb1.gallery.hd.org/_c/light/_more2003/_more...
So there's quite a lot of kovar in contact with very thin tungsten.
The thick metal conducts the heat away and the tungsten at the point where it touches the kovar is relatively cool. the kovar is cooler still.
If the kovar got as hot as the bright part of the filament, it would melt- evaporation wouldn't be an issue.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The plot thickens: couldn't find any Mo in the supernatant. Neutralising with HCl gave no precipitate. Treating this acid solution with a bit of
Na3PO4 and some Na2SO3 yielded no Mo Blue.
I simulated that test with some (NH4)2MoO4 and some Na3PO4 well dissolved, then a pinch of Na2SO3, then HCl and on the latter addition the solution
IMMEDIATELY turned a very, very deep, dark blue colour: Molybdenum Blue.
That then begs the question why the attack with KOH/KNO3 after neutralising did give a bit of white precipitate, then presumed to be MoO3.
More filament supports to be snipped, maybe even a few different brands, maybe a density too... Looking for nickel. The magnetism supports Fe/Ni.
Maybe some were Mo, some Fe/Ni?
[Edited on 10-12-2014 by blogfast25]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Very interesting indeed! I saved several burnt-out light bulbs so I may have to do some tests myself.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
4 were tested, 2 with 35 % HNO3, 2 with 37 % HCl. All were magnetic, from the same brand (2 bulbs). All in test tubes on 80 C water bath.
The nitric dissolves them in about 1 minute, the HCl in about 30 min, no acid insoluble residue in either case. Not very typical of Mo, to dissolve in
HCl, I think!
[Edited on 10-12-2014 by blogfast25]
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I performed XRF analysis of metallic parts of two light bulbs, from two different manufacturers.
Bulb one:
filament: 98% W, 0,5% Fe (the rest are other metals)
filament support: 98% Mo, 1% Fe
load in wire: 78% Cu, 2% Mn, 19% Ni
Bulb two:
filament: the same as in bulb one
filament support: 96% Mo, 2% Fe
load in wire: 60% Ni, 38% Fe
(analysis of filamet supports gave also ~1% Pd, but I almost sure it is XRF device error)
Слава Україні !
Героям слава !
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Obviously some are mainly Mo, some are not.
I can't find any Ni in my ferromagnetic ones. But I haven't got any dimethyl glyoxime...
The initial bit of white precipitate I obtained (with KOH/KNO3 fusion and neutralisation) may have been due to a bit of filament W pinched
into the filament support.
[Edited on 12-12-2014 by blogfast25]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
kmno4, thanks for posting the XRF analysis.
Just out of curiosity, instead of simply trashing them, I checked two failed incandescent lamps (one was a 100 W Osram and the other a 60 W from
unknown producer).
In both cases the lead-in wire is ferromagnetic and reacts with diluted hydrochloric acid.
The filament supports instead are brittle, are not ferromagnetic, and do not visibly react with warm diluted hydrochloric acid.
Nevertheless, these old light bulbs are a poor source of molybdenum. These wires weight close to nothing. A slightly more abundant easy source would
be the reflector caps and the sealing ribbons in automotive halogen lamps. See the manufacturers' bulletins Traditional and Emerging Applications of Molybdenum Metal and Its Alloys and Applications of Molybdenum Metal and its Alloys (for other interesting reviews from the same site, see here). At least the element collectors should be satisfied with the small amounts obtainable from these halogen lamps.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A Halogen lamp's component (the 'evaporation boat'), per one source (see page 9, Table 1), apparently contain a 'dispersion-strengthened' molybdenum
alloy (link https://www.google.com/url?sa=t&source=web&rct=j&... ). Namely, Mo-Y2O3-Ce2O3 where Y is between 0.37-.43% by weight, Ce 0-.06% and
oxygen .11 to .12%.
With respect to lamps, the heating element is at least 99.8% Mo with a small doping amount of K and Si, or contains a small amount of La2O3.
[Edited on 11-1-2015 by AJKOER]
|
|
|