| Pages:
1
2 |
Farnsworth
Harmless

Posts: 37
Registered: 11-5-2012
Member Is Offline
Mood: No Mood
|
|
Pseudo-Pentolite via ETN and TNT?
The background to the question stems from the Unconventional Shaped Charges thread on the main page. Reading about modern SCs lead me to the use of
Pentolite in many older SC weapon systems. Its properties made it rather ideal in terms of castability, VoD, handling, and brisance. PETN is of
limited accessibility to the experimenter due to the lack of available pentaerythritol, but ETN is simple enough to be made if one is careful and TNT
can be produced in small batches at fairly low cost. It is well known that ETN has rather similar characteristics to PETN for many purposes.
Could an equivalent material to Pentolite be made by combining TNT and ETN? Would it be a true eutectic combination?
Pentolite is produced by the gentle heating of pure TNT to 90C, followed by slow addition of equal parts by weight of PETN in small (5-10% total by
weight) batches and stirred gently. As each batch is added, the TNT cools and begins to solidify The TNT is then reheated to 90C and more PETN added
with stirring. The cycle is repeated until the combination is 50/50 PETN and TNT by weight.
This could make a very potent explosive for small shaped charges, but I don't have mastery of the two materials to tell at a glance if this is
workable. Would anyone care to chime in?
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I tried to add small amounts of DNT/TNT to my ETN. It doesn't mix. How do you plan separating isomers and lower nitration products from your TNT? And
it wont be storage stable. What's the point in the TNT then?
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
eBay. You can buy Pentaerythritol. $52 free shipping.
Having said that .... I always wanted pentolite but TNT was the limiting factor.
I would think ETN would work just fine to make entolite.
ETN might be a tad bit better as it has positive OB.
[Edited on 20-2-2013 by Motherload]
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
Farnsworth
Harmless

Posts: 37
Registered: 11-5-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ral123  | | I tried to add small amounts of DNT/TNT to my ETN. It doesn't mix. How do you plan separating isomers and lower nitration products from your TNT? And
it wont be storage stable. What's the point in the TNT then? |
Urbanski in Vol 4 gives the basis for purification of industrial TNT via the stellite process and washing. I don't recall it being anything
unreasonable for a laboratory setting. Further I don't think the existent isomers and remaining (.02% IIRC) impurities would be a factor if they
don't inhibit a combination with PETN (correct me if I'm wrong).
Full disclosure - I've made lab TNT only once.
[Edited on 20-2-2013 by Farnsworth]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
You are right, with the storage issues of ETN, the side products of TNT are's so much an issue. Why the military separates isomers? It's because one
or two of them aren't as stable or what? For that thing I'm a fan dinitrobenzene. Ultra insensitive and may be the most storage stable home made
explosive ever.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Entolite Production
I made a small amount of 50/50 Entolite this morning by the co-precipitation method, which is briefly described in "Military Explosives" for producing
Pentolite. Two separate solutions of approximately the same volume were made; 2.05g of ETN was dissolved in 10mL of acetone and 2.05g of TNT was also
dissolved in 10mL of acetone. Fine, low bulk density, TNT which had precipitated after the methanol recrystallization solvent had mostly cooled during
TNT purification was used. The two solutions were pipetted into about 150mL of vigorously stirred room temperature (was colder in my case) water at
the same rate in a slow stream of drips. The Entolite precipitated immediately. Pentolite is undoubtedly superior to Entolite in most ways, but
Entolite is still interesting and will most likely produce great base charges, although with less storage stability than Pentolite. According to the
usual sources, Pentolite's sensitivity is between that of PETN and TNT and this should also be the case with Entolite (sensitivity between ETN and
TNT).
Once it dries I am going to try a gram or two in a compound cap.
Ral123,
I believe some of the isomers and lower nitrate nitrotoluenes can form low melting point oils that can exude/separate from a TNT charge and can be
very hazardous, for a number of reasons, for some military applications.
[Edited on 2-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The military simply don't rely on unpredictable mixes of isomers...they prefer to have a pure enough compound in a way to get reliable
physico-chemical properties from batches to batches. This is most important for detonic properties and storage of military ammo's.
ETN/TNT mixes are very interesting and may prove to be more powerful than PETN/TNT mixes!
Because density of ETN is 1.72, it may display a slighly lower VOD than PETN (d=1.76); but on the other hand it displays a positive OB (one extra
oxygen atom per molecule) that will help burn the exces carbon rich TNT so the energy output will be higher for a given composition (probably the
perfect OB mix) and this might increase VOD and brisance of Entolite (Tetrolite) vs Pentolite.
O2NOCH2-CHONO2-CHONO2-CH2ONO2 --> 4 CO2 + 3 H2O + 2 N2 + 1/2 O2
CH3-C6H2(NO2)3 --> 3 CO2 + 4 C + 3/2 N2 + 5/2 H2
So the perfect OB mix needs 10.5 moles of ETN for 1 of TNT!
Sole problem ETN is about 10 times more shock sensitive than PETN and less stable towards heat because it is a viccinal nitric ester
(R-CHONO2-CHONO2-R with R= H or alkyl).
If there is a quest for higher densities, VOD and densities...
One would have to play:
-with MHN (mannitol hexanitrate) instead of ETN (denser, higher VOD and more extra oxygen)
-with TNB (trinitrobenzene) instead of TNT (denser, higher VOD and slighly better OB)
-with TNN (tetranitronaphtalene) instead of TNB (denser, higher VOD)
[Edited on 2-12-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I believe what you are saying about TNT purity is true, but I believe there are other reasons that may even be more important. I would have to dig for
references, but I know I have read in several places that TNT oils, from isomers and lower nitrated nitro-toluenes would leak into moving parts of
guns, etc, and pose very serious hazards. Exudation of these oils could also create voids in a shell which is a real hazard, particularly on setback.
There were other physical reasons as well why TNT with much of these impurities was incompatible with a lot of military equipment (guns, etc).
Interesting that Entolite/Tetrolite could be more powerful than Pentolite. I think a lot of the great properties of the 50/50 mix would be lost if the
mix was oxygen balanced however. A 50/50 mix will probably have a very desirable level of sensitivity to initiation. I didn't think that the
difference in shock sensitivity between ETN and PETN was really all that great. From what I have read, ETN is more sensitive but only tens of
percentage points more, if that. The big reason PETN is so much more preferred, from what I understand, is because of its greater storage stability;
it has a different structure altogether that ETN and NG and the other common nitric esters.
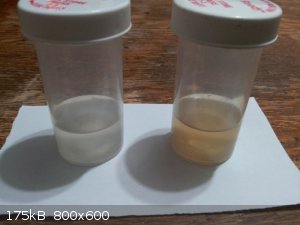
Here is a picture of the dry yield. It dries very quickly, which is great. The dry mass is 4.04g, which means that about 0.06g from what was started
with was lost, which is very little. A bit was lost during filtering and transfers and a tiny amount would have been dissolved in the water.

[Edited on 2-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Do you think the precipitated entolite is as homogeneous as a cast mixture Henning Brand? To fully utilize the extra oxygen in ETN, the molecules
should be pretty much perfectly mixed.
PHILOU Zrealone, I ran some calculations on your proposed mixtures. It's interesting how similar performance ETN, PETN and MHN gave. PETN compensates
the negative OB with higher density to some extent, but MHN gives best results thanks to its better oxygen balance.
| Code: | Mixture(50/50) Density (g/cc) OB VoD (m/s) Pdet (kbar)
ETN/TNT 1.66 -34.3% 7650 274
PETN/TNT 1.68 -42% 7600 276
MHN/TNT 1.66 -33.4% 7720 278
ETN/TNB 1.74 -25.5% 8140 313
PETN/TNB 1.76 -33.2% 8020 311
MHN/TNB 1.74 -24.6% 8200 317 |
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
1.0g of Tetrolite Initiated; 7.6mm id Al, 0.30g LA (Unreinforced Configuration & ca. 6000psi Loading Pressure)
PHILOU, I think I prefer your name for this material (Tetrolite).
I am very impressed with this material. Just 1g of Tetrolite produces results which are very comparable to the best results I have obtained with 2g of
picric acid, as can be seen by viewing the witness plate shots below.

The hole on the right, in the witness plate shots, is from this test.
 
Dornier 335A,
I think how well they are incorporated would vary with technique. I may have a closer look under a microscope, but to the naked eye it looks just like
a single compound has been formed (solid light pink color through the material). This method was one of the standard military methods for producing
Pentolite, according to the text "Military Explosives", which says a lot in my opinion.
[Edited on 2-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Not quite sure which hole is the one of most recent interest!
How about we take up a collection to buy this man a supply of nice, uniform (single use) witness plates? Least we could do in return for for the
interesting posts...
Also: Is it possible the repeated shocks by many tests to that steel disc could be work hardening it or otherwise changing the target's
characteristics meaningfully?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I always try to put the last test hole about the middle of the picture, vertically, and to the far right. Also the most recent hole has no rust. I
think you are right about the explosive hardening. I wondered about that back when I was doing the DDNP tests, but was unsure of how significant it
really was and wanted to keep using the same piece for comparisons. Yeah, one way or another it is just about time for a new witness plate. I think I
have two or three more of those same ones (undamaged) if I can find them.
Edit:
BTW, I jumped the gun a little on proclaiming the Tetrolite dry. I weighed it this morning and in the last 14-16 hours the weight of what is left
dropped an amount that would translate into the entire sample losing another 0.10g. It was actually obvious during the processing that a fair amount
of small particles were lost, stuck to the reaction vessel, etc. They could have been recovered, but it would have been more bother. Losses are
usually greater when handling smaller amounts of materials.
Here is a picture of what is left of the Tetrolite (slightly less than 3g). It should be very close to the equilibrium moisture content now. Notice
how it has taken on a lighter color as well.

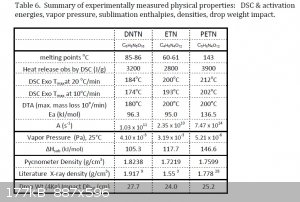
Here is a table of experimentally determined physical properties for ETN and PETN taken from the article, "Characterization and Analysis of
Tetranitrate Esters". Dh50 is the height at which there is a 50% probability of explosion. ETN is only slightly more sensitive to impact
than PETN, however, it really falls short of PETN in terms of heat release.
[Edited on 3-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I'm curious to hear what you see under the microscope. I have coprecipitated mixtures before and the crystals formed were about 5 µm - not
particularly homogeneous that is. That was by pouring a hot concentrated solution of potassium chlorate and potassium ferricyanide into cold ethanol.
The particle size could probably be reduced further by spraying a mist of the solution into the ethanol and possibly using a more dilute solution, but
still.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I will have a look under the microscope, but I am not convinced that it is imperative that mixing be that fine. I have attached a jpg image of a
snip-it on Pentolite production from "Military Explosives".

Also from "Military Explosives", "The minimum detonating charges of lead azide and mercury fulminate required for pentolite are intermediate between
those for PETN and TNT and are very close to that of tetryl."
[Edited on 3-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have to point out that in the co-precipitation method you should mix the solutions of TNT and (P)ETN before you add them to water
to precipitate the solid explosive. This will give you an even more uniform distribution.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
According to Fedoroff, only 20% of the PETN dissolves in the molten TNT. I actually thought all of it dissolved. Anyway, it shouldn't matter too much
how well the two compounds are mixed as both are oxygen negative. Entolite will of course work well too even with rather large particles. But to get
the maximum performance (especially brisance), the extra oxygen in ETN should be utilised by the excess carbon in TNT as quickly as possible.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks Microtek, that would make the process easier as well. I now see that it actually does say in "Military Explosives" to mix the two solutions
before adding them to the vigorously stirred water. Microtek, do you know how much acetone and water should be used for the co-precipitation method? I
was unsure of how much to use. I was feeding the two solutions of about the same concentration in at approximately the same rate, but mixing them
beforehand would be much easier that is for sure. Interesting to note that in the more modern slurry method, of pentolite production, preformed
particles of PETN are used which are then coated with TNT. So the better particle size control of this more modern slurry method was deemed more
important than the more intimate mixing of the co-precipitation method. I assume that the co-precipitation method could easily achieve a more intimate
mixture of the two components though not as much uniformity of mixing and particle size.
Dornier 335A, I imagine that degree of mixing can be very important, but I imagine that it becomes less and less important as you get finer and finer.
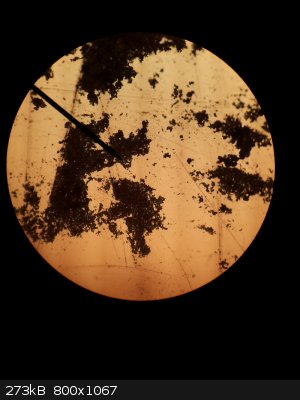
It would depend on intended use as well how important it was. I am not exactly sure how to quantify it though. I took a microscope picture at 100X
magnification, but with a bottom light I had to flatten the sample out with the back of a spoon so the light could get through and make it visible.
The sample was on a piece of clear plastic also (I have glass slides somewhere). I think you can get an idea of how fine the crystals are by looking
at it, but it would be better if a scale was there.
According to "Military Explosives", 87% TNT & 13% PETN form a eutectic with a melting point of 76.7C. So 50/50 pentolite when cast has about 42.5%
PETN and about 57.5% of the eutectic mixture. So in this case about 15% of the PETN dissolves.
[Edited on 5-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I would be interested in seeing the "Entolite" further characterized-
Melt point?
Impact tests, even if so crude as hammering a few milligrams on a steel surface?
Friction test, ditto.
And as a quick & dirty test- wrap perhaps 100mg of "entolite" tightly in several layers of Aluminum foil, place on a thin sheet metal witness
plate and place a disposable alcohol burner or small torch head below the plate in a fashion that will cause the sample to be slowly heated to
deflagration temperature. ETN reliably undergoes transition to detonation under these conditions, what would the co precipitated or melt cast mixture
do?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Good idea Bert, I will see about doing a few tests in the next little while. Anyone else is welcome to test some as well of course.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Mainly curious about safety, one had best assume it to be at least as touchy as straight ETN until data is collected-
Also: If people were going to consider this as a melt cast filler, it would be well to check how shock & friction sensitive the liquid mixture is
BEFORE choosing to expose yourself to risk of an accident... Can anyone propose a method to determine that. Impact testing of a melted sample- How
best to conduct such a test???
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
That was the main reason I chose the co-precipitation method of forming the explosive. I am sure ETN can be melted safely, but I have heard or read a
lot of scary stories and wanted to avoid melting it if I could.
Ok, I just did the first crude test. A bit of the loose powder, in amount less than the size of a paper match head, was put in one fold of aluminum
foil, put on an anvil and struck hard with a ball peen hammer. The sample was hit several times, with fairly solid shots, which did not result in
explosion. To put it subjectively, the blows were maybe not what would be needed to set off picric acid, but they were not too far below either. I
would need to get or build a precise apparatus to get any kind of accurate quantification of impact sensitivity, but the sample appears to be
significantly less sensitive than ETN to hammer blows which is no surprise.
[Edited on 5-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  |
According to "Military Explosives", 87% TNT & 13% PETN form a eutectic with a melting point of 76.7C. So 50/50 pentolite when cast has about 42.5%
PETN and about 57.5% of the eutectic mixture. So in this case about 15% of the PETN dissolves.
|
I did a little reading on eutectics. Hopefully this explanation is more or less correct. I should have said the composition of what solidifies last,
and at the eutectic temperature, from the melt contains about 15% of the PETN. More than 15% of the PETN may dissolve and be part of the melt above
the eutectic temperature, but any PETN dissolved above that amount crystallizes out before the temperature drops to the eutectic temperature. Once the
temperature has dropped to the eutectic temperature the temperature and composition of the melt, and consequently the composition of the solid being
formed, does not change as the melt solidifies. Once the eutectic mixture has completely solidified the temperature will begin to drop again as the
solid cools.
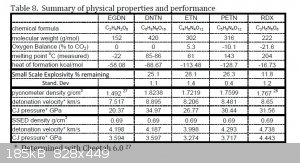
Also, the physical properties table posted several posts up had DSC heat release values displayed. The DSC kinetics values found in the study and
displayed in the table were there mostly to make stability comparisons. They found PETN to be more thermally stable; for details the study can be
easily downloaded from this forum or elsewhere online. Here is a table which contains more of the performance related information, from the same
article. The values were determined using a computer software called Cheetah.
[Edited on 7-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
thickness
This is the hole from one gram tetrolyte? It is labeled correctly? What is the thickness of steel? I do not see written anywhere. 3 mm or 1/8 inch?
Thank you, LL .....

|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Tetrolite Melting Point
Melting point of the eutectic portion of the 50/50 tetrolite was found to be about 45-46C, which is far below the melting point of ETN (~60-61C) or
TNT (~80-81C). At that temperature there was still a small amount of un-dissolved material, but it appeared to be less than 10% of the total sample
under test which is just a crude estimation based on site. After running the test several times it looks as though all solid becomes part of the melt
at only a couple degrees above the first main melt temperature (eutectic temperature). The melting point is far too low for this explosive to be of
military use. More sensitivity testing will need to be done, but it would likely be perfectly safe to cast for amateur charges. The low melting point
would normally not be a problem, and possibly an asset, in an amateur application (I think).
LL,
Thickness of witness plate is only about 2.6mm; I have been using the same plate for so long that I didn't think to give specifics again. Yes, that is
the correct hole. It is the only one without a layer of rust covering the exposed steel surfaces so it is easy to tell. The exposed steel accumulates
rust very quickly. The loading pressure could have been much higher for the tetrolite base charge which likely would have significantly improved
performance. All the other holes were made with picric acid base charges, except one shot made with an improperly primed TNT base charge which did not
perforate the plate.
[Edited on 7-12-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
That witness plate has really been subject to an extensive amount of sustainable exploitation 
Bet you can squeeze in at least one more test with that perforated plate though 
Exact science is a figment of imagination.......
|
|
|
| Pages:
1
2 |