| Pages:
1
2
3
4
5
6
..
10 |
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
There is one plausible way of neutralizing nitrocellulose I've been thinking about. A dilute acetone solution of nitrocellulose poured into water will
precipitate the NC as very fine particles and threads. If the water has been made slightly alkaline with sodium carbonate for example, all the acidic
residues inside the fibres should be neutralized.
I know that Plante1999 recommended boiling nitrocellulose in dilute urea solution instead of sodium carbonate. The nitrocellulose is then washed with
water and one last time with methanol before drying.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I am not sure why urea would be superior to sodium carbonate except that maybe traces of alkali left in the nitrocellulose could cause decomposition
of the nitrocellulose in storage and trace amounts of urea left could act as a stabilizer. However, for colloided nitrocellulose powders (modern
smokeless propellants), urea is apparently not a suitable stabilizer. From COPAE, "Urea is used in dynamite and in celluloid. It reacts with nitrous
acid to produce nitrogen and carbon dioxide, and is unsuitable for use in smokeless powder because the gas bubbles destroy the homogeneity of the
colloid and affect the burn rate. The small gas bubbles however commend it for use in celluloid, for they produce an appearance of whiteness and
counteract the yellowing of age."
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
@Hennig Brand you said your material degraded for months. A sample made with WFNA/H2SO4 could burn very violent after more then an year and quite a
time at elevated temperatures. It was only washed with tap water. Could it be that the highly nitrated is more stable?
I wont consider urea as a stabilizer because it releases water in it's reaction and water degrades NC. IIRC highly nitrated NC can detonate in a thick
drinking straw. Witch I suppose makes it usable for base charge.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
In most instances even after a couple years the material was still usable as propellant, but it was noticeably weaker than freshly made
nitrocellulose. In a couple instances the material was extremely degraded and was very weak in comparison to freshly made nitrocellulose. Storing
nitrocellulose in a sealed plastic bag, rather than letting it breath, seemed to greatly increased the rate of decomposition.
If you used mixed acid to produce your nitrocellulose it is unlikely that your product was that much more highly nitrated than mine. With a mixture
of sulfuric and nitric acids the maximum level of nitration is around 13.4-13.5%N IIRC. Unless my estimates have been very inaccurate, I have produced
nitrocellulose with a level of nitration of up to 13%N or so using ca. 91% sulfuric acid and a nitrate salt. Using nitric acid instead of a nitrate
salt is supposed to produce a more stable product, however, because the product has less impurities.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have been spending a little time looking into purification of nitrocellulose for long term storage stability. As was just discussed, putting less
impurities into the reaction mixture, by using nitric acid instead of a nitrate salt, is probably the first step since it is likely easier than trying
to remove the impurities post reaction. I have made several batches of microcrystalline cellulose, but have yet to nitrate any of it. If it is as easy
to purify/stabilize as it is reported to be it may be the answer, at least for the hobbyist. I have also been experimenting a bit with cutting up,
thrashing and poaching nitrocellulose made from cotton balls. A blender was used to do the thrashing. A set of kitchen shears was used to cut the NC
batt into small chunks so that there would be no long pieces of fiber to wrap around the drive shaft and gag/burnout the blender. The blender was run
on high speed and only for about 30 seconds, but it was obvious even from the short run time that it was thrashing the NC more than cutting it up. The
thrashing did, however, seem to separate the fibers and fluff up the NC a lot as well as tear a lot of the fibers into shorter lengths (pulling the
clumps apart between the fingers showed the shortened fibers). The NC also took on a much lighter color indicating that a lot of the junk from the
drain cleaner acid and fertiliser grade nitrate had been released or shaken free. The next step was poaching; a short 20 minute boil was performed in
a 2% sodium bicarbonate solution and then another 20 minute boil in clean water. All of these times are extremely short, and were only done as a quick
test to judge the suitability of the process. Although, often times the greatest proportion of benefit comes from the first bit of time. During the
thrashing and poaching about 0.34 grams of the about 15 grams of nitrocellulose was lost as fines.
From the document on military nitrocellulose specifications, attached below, it is obvious that nitrocellulose is cut/beat to a very fine form in
industry (ca. <0.56mm fiber length). I could have easily cut the nitrocellulose up much finer in just a few minutes with scissors and the blender
could have been run much longer, but I am sure that this is not the best way. I am still exploring the options, but there must be a motorized
household implement, like a food processor maybe, that could perform an adequate job of nitrocellulose comminution (particle size reduction).
      
Attachment: Nitrocellulose Detailed Military Specifications MIL-DTL-244B.pdf (112kB)
This file has been downloaded 1426 times
[Edited on 21-11-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Ran out of room.
Microcrystalline cellulose was made by hydrolyzing pure cotton cotton balls in a 5% HCl solution at the boiling point or just a little under. The
cotton looked to have mostly fallen apart in less than 30 minutes, but the heating was maintained for a total of 2 hours. The dry product is very
clumpy, but rubbing it between the fingers a bit shows that it is an extremely fine powder. I have yet to nitrate any of it, but it seems at this
point like it might be the key to stabilized nitrocellulose for smokeless propellants for the hobbyist.
[Edited on 21-11-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
TGT
Harmless

Posts: 46
Registered: 9-11-2014
Member Is Offline
Mood: No Mood
|
|
I have a large amount of Microcrystalline cellulose from ASA extraction when making Picric Acid. It was the only other additive in the aspirin
tablet. Do you think this would work for nitration? It is as a total powder when wet and does not clump like wet cellulose. What do you think?
TGT
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
All this talk about MCNC has got me pretty interested to try synthesizing some. Enough so that i purchased some pharmaceutical grade MCC. In the next
week or so i will attempt to nitrate a small amount of it using a standard WFNA/sulfuric bath and will attempt to get it highly nitrated which should
not be too difficult going by what i have read about the process involved from the Microcrystalline cellulose nitrate thread a few pages in from this
one and the patent by a user here, Ordenblitz. although i wont be using it as a propellant since i have no firearms but i will attempt to detonate
some in medium confinement and a with a reasonable cap. will see how it goes and i ll keep a nice record to post of my experiments with it.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
If it is microcrystalline cellulose I imagine it would nitrate just fine.
I guess I didn't describe the homemade MCC from above correctly. It dried in clumps, but once broken up it stays as a powder, unless pressed. Yeah, it
would be interesting to see the results of some tests with MCNC; it might just make a good base charge for detonators among other things. MCC can also
be nitrated to a bit higher level of nitration, since there is the possibility of increasing the ratio of nitrate groups to cellulose monomers (more
locations to add nitrate groups).
Here is an interesting document I found online giving an overview description of a, military specification, nitrocellulose production process.
Attachment: Nitrocellulose - Military Specification Production Process.pdf (202kB)
This file has been downloaded 2251 times
[Edited on 23-11-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Cellulose Nitration Diagram & Spreadsheet
Here is an Excel spreadsheet for determining the approximate quantities of acids of various strengths required to obtain nitrocellulose of any
strength (%N) desired. A couple of ideas were borrowed from the PETN Excel sheet that was posted many years ago and is still in circulation.
It is not completely automated and requires that a certain amount of iteration be performed by the user. The boxes in bright yellow are variables
which can be changed. Changes are made until the post nitration mixture (end acid composition) matches up with the diagram for the %N chosen.
The nitration triangle diagram was taken from, "High Explosives and Propellants", by Fordham (pg.39).
It is very obvious that using 65% HNO3 and 91% H2SO4 is a very wasteful way to try and obtain NC of high percent nitrogen.
It may require changes, all input is welcome.
Attachment: Cellulose Nitration Diagram & Spreadsheet.xlsx (129kB)
This file has been downloaded 1194 times
Attachment: Cellulose Nitration Diagram & Spreadsheet (compatibility mode).xls (143kB)
This file has been downloaded 1214 times
[Edited on 20-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Dear Lord, it's a one eyed, one horned, frying purple cotton eater 
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
If one gets the nitration acid composition really wrong or fails to remove adequate heat it could definitely eat some cotton. 
Do the numbers seem ok?
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
What's everybody here made? This is a some speciality of cook chef? I see, that photos are older, but I must be carefully what's the matter...
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I assume that this is a troll, but I will answer anyway. Some chemists, even the amateur ones, would get angry if you compared their activity to
cooking, but there are often a lot of similarities I find. I happened to be away from home, and got bored, and the kitchen implements used in the
above photos were all I could gather together in order to hydrolyse some cotton. I think the results were the same as they would have been if I had
been using laboratory grade glass and a laboratory grade hotplate.
Regular nitrocellulose is very difficult (especially for the amateur) to make storage stable because its physical form is such that complete removal
of residual acidity and other impurities is very difficult. Microcrystalline nitrocellulose is in a form that is much easier to remove impurities from
and make storage stable. Propellants can degrade quite quickly if not properly purified and stabilized; best case scenario the propellant losses
performance, worst case scenario the propellant ignites and burns or explodes and causes death and destruction.
I am just evaporating the acetone from a fresh batch of double base propellant based on microcrystalline nitrocellulose, so maybe tomorrow I will test
some of it.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NC
Henning, thank you. Microcrystalline cellulose, or very short fibers is an important raw material for the esterification. To ensure the best possible
neutralization. Thus stability. Understood. I am curious about the tests.
... ...LL ...LL
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
You are right about stabilizing the MCNC. the small batch i recently made had since decomposed quite dangerously. The container was full of the
dreaded red gas. i thought it was washed and neutralized very well but i guess it was not good enough. Quite unnerving to find it in that condition. I
immediatley disposed of it. it also was not stabilized in any way. Also the area it was stored gets pretty warm so that no doubt accelerated the
decomposition.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
MCNC/NG, 60/40, Double Base Propellant Testing
MCNC should be much easier to stabilize than regular NC. I have had MCNC and MCNC double base propellant in storage in plastic sandwich bags for a
couple of months now and they show no signs of decomposition. After nitration the MCNC was well washed to remove nearly all residual acidity and then
boiled in a 2% sodium bicarbonate solution for 10-15 minutes followed by another 10-15 minute boil in plain water. I have diphenylamine now, but I
have yet to use any to stabilize a propellant.
I tested some homemade double base propellant this morning that was made from MCNC and NG. The performance was not as good as when regular NC was used
and I am not exactly sure why. Double base propellant made with MCNC has much higher bulk density than DBP made with regular NC; 0.1g in the .22LR
cartridge only filled it about 60% full whereas it would have been full to the brim if regular NC had been used. Higher density perhaps coupled with
larger average particle size could have been the cause of lower performance. The other possibility is that MCC is more difficult to nitrate to a high
percent nitrogen than cotton. The homemade MCNC DBP is still very serviceable propellant however.
The home made propellant is MCNC-NG, 60-40, and was made the same as the last propellant, based on regular NC, using acetone as the solvent. The home
made propellant produced a muzzle velocity of 1166fps, while the commercial propellant produced a muzzle velocity of 1228fps (0.10g of propellant in
both cases). The accuracy of the scales could have accounted for the difference in performance between the two, but there did also appear to be more
dark colored fouling in the cartridge which held the homemade propellant (even a couple bits of unburned propellant could be seen). The MCNC
propellant may require a stronger primer. At any rate the homemade propellant is very serviceable.
No stability testing has yet been done other than observing small samples held in regular storage.
     
[Edited on 24-1-2015 by Hennig Brand]
[Edited on 24-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
What were the conditions during which the acetone solvent was evaporating? Is it possible it dried at a slow rate and you shot it too soon?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I am impressed. Do you even have time to show up at your day job?!
Have you looked at the information on "nitrometer" testing given in Naoum and Tenney Davis? I am curious as to the results from your microcrystaline
NC.
Cellulose is an odd molecule, the length of chains is a major factor in physical behavior of lacquers and varnishes used for painting and coatings.
Partial treatment as you have done to create MCC is use to alter viscosity of lacquers- When made with same solids content of NC & identical
carrier solvent, the shorter the chains, the lower the viscosity. Allows a smaller droplet size in spray coating with same film build/volume of
lacquer-
I would bet it has some effect on both nitration and thermal decomposition profile in propellants.
-------------
Did you disassemble and weigh propellant charge from one or more rounds of factory ammunition, then use the case(s) and projectile(s) to load the home
made propellants? Followed by firing another (unmolested) round, or rounds from same lot of cartridges to establish factory round velocity?
If you are firing an "as factory assembled" round of .22 LR in comparison to your reloaded round, the mechanical differences induced to the reloaded
round by your processing is a factor.
If I've guessed your test procedure correctly, try this again but treat both rounds EXACTLY the same way... Pull the bullet from the factory round and
then put it back, the damages to bullet heel, loss of lubricant in handling and other small changes could account for 100 fps of velocity!
Also: unburnt grains of propellant usually equals too low operating pressure (damage to bullet, poor bore obturation (sealing)?)
Another factor in unburnt powder: A too large/non uniform grain size. If you have the capability, FLATTEN the powder. Just run the powder through a
precisely aligned set of heated rollers? At least one dimension of each grain is then guaranteed to be the same, so burning time in that axis at least
is uniform. A lot of ball powder is treated this way, giving a flattened ball profile to the grains- This improves uniformity of burning speed,
regardless of non uniform grain weight (caused by variations in ball Dia.)
That simple little .22LR cartridge is a fiend to make uniformly, even in a professional factory setting. Witness the +100-500% difference in the cost
of competition grade ammunition to cost of plinking grade .22 ammo! And the common practice of rifle teams, buying multiple cases of different lots of
the same brand .22 ammo, testing, then reserving only the best lots for competition.
Larger centerfire rounds with jacketed bullets are more forgiving of tiny variations, if you have that capability. Perhaps even a muzzle loader of
larger caliber would be more suitable to assess propellant differences, if it were possible to load exactly uniformly as far as depth of bullet
seating, % of powder volume in area below bullet, bore condition, bullet hardness... All the minutiae of loading parameters.
Finally, what brand of .22 LR ammo are you using for the test case/primers? If it is a plinking/hunting grade, the less precise priming of such ammo
can give a spread of velocities equal to what your single test showed here- In totally unaltered samples taken from the same lot/case/box!
[Edited on 25-1-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
RoXefeller, good thinking, but after being cut into flakes the propellant was given a couple of days spread out at room temperature to get rid of
nearly all the acetone.
Bert, thanks for the very informative reply.
I have read about nitrometers a few times, but thought at the time that they were complicated and specialized. Perhaps I should give them another
look.
You are correct, the ammo is cheap plinking ammo called American Eagle, put out by Federal. I have tested many rounds and it is common for every
second or third round to be as much as 20fps, or even higher in a couple cases, than the average. The way I am pulling the bullets is horrible; the
bullet is wrapped in multiple layers of paper and then griped with vise grips and then pried and pulled out. I have had to throw out some bullets
because they got significantly damaged while trying to remove them from the casings.
"Did you disassemble and weigh propellant charge from one or more rounds of factory ammunition, then use the case(s) and projectile(s) to load the
home made propellants? Followed by firing another (unmolested) round, or rounds from same lot of cartridges to establish factory round velocity?"
Yes, that is exactly what was done. I have weighed half a dozen or more from the same box and the load was reasonably consistent, at least as far I
could tell with the accuracy of my scales. The commercial load weighed between 0.09 to 0.10g in those measured. Obviously the accuracy of the scales
are a real problem here. I did just get a set of scales which will give me another decimal place though. Also, your suggestion to pull the bullet from
the factory load to be tested and weigh the powder before reseating the bullet is a good one. This would help ensure that the powder load weight was
the same for both the commercial and homemade tests and if done carefully help ensure that the bullets and casings were in the same condition before
the test.
I may look into a set of rollers. Seems like a cheap and easy way to ensure at least one dimension of the propellant flakes are the same, as you
suggest.
The .22LR was chosen because it was cheap and readily available to test and thought to be safer than performing tests with a larger caliber rifle.
You are right though, it is much more difficult to get accurate test results with the .22LR for many reasons. I imagine that everything, including the
primer load, has lower tolerances in a .22LR plinker than in a decent center fire rifle round. Larger rifle rounds really would absorb much better
small differences in power weight and other loading specifics (percent differences are much smaller for same inaccuracies).
[Edited on 25-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
Don't know your personal collection, but revolvers can be overloaded and work pretty well. I've fired 44mag out my ruger filled to 100% with ball
powder. But you could also down-load. If you had either a 44mag or 357mag, you could look up the grain loads for the 44spl or 38spl and load for
those. You would have a normal cartridge, but your firearm would have strength margin in case the load was stronger than anticipated.
Another thought is the ballistic modifiers used in the NC based propellants. It makes the burning rate of the propellant smoother, so it doesn't burn
powder too fast at high pressures.
Either way, 1100 or 1200 fps, you're correct, it is a very serviceable load. Too bad single base rifle propellant would be crazy to form.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
MCNC/NG, 60/40, Double Base Propellant Testing in 30-06 Rifle
This morning I dug out a 30-06 Winchester rifle and some old cartridges to do some further propellant testing. The propellant used was from the same
batch as was used in the last .22LR test. I had an assortment of old 30-06 rounds, but several of them looked to be the same. The bullets were
carefully removed from two rounds using the method described here:
https://www.youtube.com/watch?v=XVaE_LKmjPc
Basically an empty casing of the same type was slid over the bullet and rotated around with some hand pressure to loosen the bullet and then the
bullet was pulled out by hand.
The bullets and powder loads were weighed. One was reloaded with the full commercial load and another was reloaded with homemade double base, 60-40,
made with MCNC. The bullets weighed ca. 150 gr (ca. 9.7g) and the loads of commercial propellant were ca. 45 gr (ca. 2.9g). On reviewing loading data
pages it looked as though single base tubular propellants were often used to load 30-06 shells. Since the homemade propellant was 60-40 double base,
flake propellant, it was decided that a lighter load should be tried first. The load of homemade propellant weighed out was 2.5g, but only 2.0g was
used since because of the lower bulk density, relative to the commercial tubular propellant, 2.0g filled the casing up to the neck.
I am glad I didn't use a heavier homemade powder load, the 2.0g of homemade propellant produced a muzzle velocity just slightly less than the 2.9g of
commercial propellant (2631fps versus 2647fps).
Bullet weights: 9.7g
Commercial propellant load: 2.9g tubular SP (single base?)
Homemade propellant load: 2.0g double base, 60-40, flake (based on MCNC)
Muzzle velocity commercial propellant: 2647fps
Muzzle velocity homemade propellant: 2631fps
     
[Edited on 30-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Impressive results! I suppose the primer was stronger this time and/or the heavier bullet helped the propellant to burn more complete.
Have you estimated the nitrogen content of your MCNC?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|

You are fairly new to reloading. The test you described made my hair stand up, I hope you were not HOLDING the rifle!
Extruded powders used in medium bore bottle necked cartridges like the 30-06 are usually single base, as you guessed. Your 60:40 powder is probably a
LOT hotter burning.
Your uncoated grains are likely degressive burning, the perforated tube grains would be close to neutral burning, and probably coated with deterrents
to effectively give a progressive burn.
I would bet your propellant is producing far higher than design pressure for 30-06 early in the burn, please post clear pictures of the cartridge
heads of the factory and experimental round? How do the primers look, is the home made propellant one more flattened! Did primer cup metal flow into
firing pin hole, pick up all the little details of bolt face more so than factory round? Did case head flow brass into the gun's extractor cut out,
was it harder to extract experimental round?
If you have a rifle that can be sacrificed to science, put a strain gage on the barrel and are firing from a protected, remote location- Something
more might be learned. If not, I'd stop.
I will try to assemble more information on how commercial powders are tested.
Interior ballistics
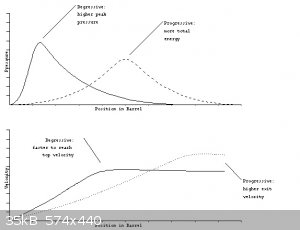
[Edited on 30-1-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
If it would make you feel better I could tell you that I wasn't holding the rifle, but I would be lying.
The empty casings look basically identical. The casing for the homemade propellant did not seem any more difficult to remove after firing. Primers
look identical. I am indeed fairly new to reloading, but I read enough beforehand to know that I was taking a risk.
The casing on the right in the pictures is the casing which held the homemade propellant.
   
[Edited on 30-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
2
3
4
5
6
..
10 |