| Pages:
1
..
11
12
13
14
15
..
20 |
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
In my experience a caramel odor is often a tell-tale of formate salts or very dilute formic acid.
Dakin's original experiments did not exclude oxygen, indeed as I recall he sparged his solution with air in order to speed the removal of the
acetaldehyde that was formed. But his reaction was conducted at or around 40C. It's also possible that ammonium lactate is less dissociated than other
lactate salts and that this would affect the speed of various competing reactions.
His original publication is in J. Biol. Chem., 1906, 1, 171. For some reason I can't find this online any more, although a followup paper that he
published in the same journal in 1908 is available via Google Books.
The less you bet, the more you lose when you win.
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by bbartlog  | | His original publication is in J. Biol. Chem., 1906, 1, 171. For some reason I can't find this online any more, although a followup paper that he
published in the same journal in 1908 is available via Google Books. |
Is this what you are referring to?
http://www.jbc.org/content/4/1/91.full.pdf
http://www.jbc.org/content/1/2/171.full.pdf
http://www.jbc.org/content/4/2/227.full.pdf
[Edited on 16-10-2013 by WGTR]
[Edited on 16-10-2013 by WGTR]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Interesting. Yes and no; clearly that is the reference I gave (well, the second one is), and it's mostly on topic, yet none of those three is exactly
the paper I remember. Maybe someone other than Dakin did the first piece of work. I'll have to backtrack through the references he gives when I have
some time and see whether I can turn it up. It's definitely from that era.
The less you bet, the more you lose when you win.
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I'm just going to post this reference for right now; it seems very detailed and useful. I
don't think it's been posted before.
The Catalytic Partial Oxidation of Ethyl Alcohol in the Vapor Phase
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
Just made acetaldehyde using Rhodium's method. Got (what looks like) a good yield of acetaldehyde and possibly a slight amount of ethanol. I am too
lazy to form the trimer for purification, so I just dried it and called it a day!
http://www.erowid.org/archive/rhodium/chemistry/acetaldehyde...
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
I tried something else with less expensive metals/compounds as silver and aluminium oxide:
reduction : CH3CH2OH --(H2SO4(>150*C)/-H2O)--> H2C=CH2 (ethylene)
oxidation : H2C=CH2 + Air --(Ag powder deposited on active charbon/azbest at 200-300*C)--> (CH2-CH2)O (ethylene oxide)
transposition : (CH2-CH2)O --(Al2O3 powder deposited as silver, at 200-300*C)--> CH3-CH=O (ethanoic aldehyde)
and seems to work...
also I can obtain ethilene oxide, through this process, wich is a extremly useful reagent 
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
also I know an other good method to make the epoxide of ethylene in large quantities and in a relative small amount of time... but is much more
complicated and involve an other intermediate : 2-chloroethanol (Cl-CH2-CH2-OH)...
|
|
|
Crypto
Hazard to Self
 
Posts: 50
Registered: 18-11-2013
Member Is Offline
Mood: No Mood
|
|
DoctorZET can you elaborate a bit on your method? What setup have you used and how have you prepared the catalyst?
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
Here is a process of making acetaldehyde:
 
|
|
|
Crypto
Hazard to Self
 
Posts: 50
Registered: 18-11-2013
Member Is Offline
Mood: No Mood
|
|
After reading the whole thread I must say that the above procedure seems to be the most practical for an amateur chemist. One could prepare the
aldehyde practicly in any needed quantity during just one run. No toxic chromates are used which is nice and I guess that the catalyst beds could be
placed one after another to omit the condensation step.
Thanks a lot Z. How did you deposit the catalyst on it's support?
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
And here is the another process:
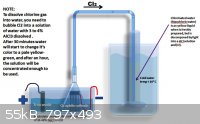  
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
And the Ag powder / Al2O3 powder can be easily spread in the glass wool:
Take a piece of glass wool and pass it over hot steam. Then put it into a closed jar with some catalyst powder and give him a really good shake.
If you have problem with the silver powder (I remember that I have actually some problems, because silver must be powdered, not granules)... try this:
Put a piece of silver into nitric acid.
After it dissolves, wash in the solution of AgNO3 some copper wool (from electric wires) until a silver film start to form on copper.
Now you have the silver powder on copper wool 
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
also you can try to make the silver mirror reaction using the glass wool in the solution of glucose/sucrose + NaAg(NH3)2 ... but I think is too
complicated for our purpose 
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Sabatier and Senderens did not use copper scrubbers. Bouveault (attached) made an apparatus improvement going vertical, and used rolls of copper gauze
coated with reduced copper, from the hydroxide and hydrogen at 300C.
Attachment: Bull_soc_chim4_3_119_1908.pdf (556kB)
This file has been downloaded 1196 times
|
|
|
Oxirane
Hazard to Self
 
Posts: 92
Registered: 19-9-2014
Member Is Offline
Mood: No Mood
|
|
I am planning to do some acetaldehyde in the future. My plan is to drop ethanol into steel tube with dropping funnel to control the feed rate. This
vapor will go to SS or glass tube that is packed with catalyst and heated to 300-400C with hot air gun.
Catalyst would be prepared by dissolving copper sulfate into water and mixing silica gel crystals, stirring and filtering out and drying and then
pre-heating to decompose the sulfate into oxide with gas flame. This oxide will be flushed with ammonia or hydrogen right prior to use to generate
active copper catalyst. This ensures maximum catalytic surface.
Unreacted ethanol will be condensed into first flask fitted with condenser. Flask stands in water and reflux condenser both are kept at 20C.
Acetalhdehyde vapors will go over this and condense to next one, where flask is immersed in ice bath with reflux column circulated with same water and
insulated to keep it cool as long as possible. From this end an exhaust tube is lead away safely if anything that does not condense comes over.
My idea is that this same setup could be used to produce also formaldehyde and diethyl ether. Ether can be made with Al2O3 catalyst from ethanol, but
cannot remember temperature right now. Formaldehyde would be bubbled into cold water which is placed into second flask but otherwise the setup will be
the same for all these three.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
i have also come up with a few reactions to make acetaldehyde .pls consider them ,we can then choose the most practical one
1.In the beginning pages ,i saw that some members were having trouble with oxidizing ethanol to acetaldehyde using hydrogen peroxide.
but ethanal can be made from ethanol using peroxide if FeSO4 is added to it .the mixture of peroxide and Fe+2 is called fenton's
reagent
http://en.wikipedia.org/wiki/Fenton%27s_reagent
2.some members suggested doing dehydration of glycol using sulphuric acid but ended up getting dioxane.
instead of H2SO4 ,anhydrous ZnCl2 can be used to get ethanal
3.another member suggested doing ozonolysis on propene ,but it is risky.
alternatively,one could pass propene through cold ,dilute KMnO4 and get propan 1,2 diol which can be subjected to periodic cleavage using
HIO4 .
even lead tetra acetate can be used if periodic acid is not available
periodic cleavage -http://en.wikipedia.org/wiki/Glycol_cleavage
lead tetracetate preparation-https://www.erowid.org/archive/rhodium/chemistry/lead.tetraa...
if propene is not available ,then propylene oxide(methyl oxirane) can be converted to propan 1,2 diol using acidic or basic treatment ,then the above
mentioned diol cleavage can be carried out
propylene oxide http://en.wikipedia.org/wiki/Propylene_oxide
sorry, after posting this link i realised that propylene glycol is directly available-http://en.wikipedia.org/wiki/Propylene_glycol
4.we could also treat 1,1 dichloroethane with base to get gem diols that immediately tautomerise to acetaldehyde
ethylidene choride http://en.wikipedia.org/wiki/1,1-Dichloroethane
5.the sommelet reaction[http://en.wikipedia.org/wiki/Sommelet_reaction could be tried(as Hexamine is very easy to make https://www.youtube.com/watch?v=d2N0zAoVYew ,although the reaction is specific for benzyl halides ,the wiki page says that some aliphatic
aldehydes have been prepared
6.finally nitroethane can be converted to acetaldehyde using the nef reaction with 70% yield of acetaldehyde
nef reaction-http://en.wikipedia.org/wiki/Nef_reaction .the yield of acetaldehyde could be increased to 90% if Fe/HCl is used instead of sulphuric acid as told
in the wiki page according to this journal-http://www.tandfonline.com/doi/abs/10.1081/SCC-200051681?jou...
if anyone is interested i will upload it tomorrow
P.S. - one member wanted to know how to get selenium dioxide for making methylglyoxal http://en.wikipedia.org/wiki/Methylglyoxal from acetone,which could then be oxidised to convert the aldehyde to acid and then heated to get
acetaldehyde by decarboxylation .
IIRC,when i was small ,we used to make solar cells from selenium wafers found in old transistor radios .but i dont think we can find those radios
nowadays
also .if the selenium oxide is used to oxidise ,then SeO2 would itself get reduced and i remember reading somewhere that hydrides of
selenium stink to high heavens ( even worse than hydrogen sulphide) ( even worse than hydrogen sulphide)
H2Se -http://en.wikipedia.org/wiki/Hydrogen_selenide
[Edited on 3-11-2014 by CuReUS]
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Nitroethane is even harder to get than acetaldehyde.
|
|
|
Oxirane
Hazard to Self
 
Posts: 92
Registered: 19-9-2014
Member Is Offline
Mood: No Mood
|
|
For most private chemists(or maybe in this context, cooks), nitroethane would be something that was more likely held behind locked doors than those
the hazardous substances legislation generally requires. Using nitroethane for acetaldehyde sounds something like making benzoic acid from
benzaldehyde.
By the way, on the topic, is it possible to catalytically oxidize acetaldyde to acetic acid? I've read on it somewhere and I remember the process is
similar to dehydrogenation, but oxygen is introduced to the reactor aside acetaldehyde. But I see there a risk of self-ignition since the reactor
conditions are sufficiently hot for that.
[Edited on 3-11-2014 by Oxirane]
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
As the end of the school semester approaches, I find myself with less and less free time in the lab. For this reason, this project is not documented
as well as I would like to have it. Along the thread of ethyl alcohol direct dehydrogenation, I'm posing some pictures of how I assembled a single
bead string reactor. It has some issues. For one, I would probably choose to replace the glass reactor tube with a similarly sized stainless steel
one. Stainless has a bit more elasticity, and is easier to bend safely. Like glass, stainless has low thermal conductivity for a metal. Also, I can
more easily braze stainless fittings together than I can fuse borosilicate glass. Thermal shock is not as hazardous with stainless, either.
I tried some initial experiments dehydrogenating alcohol with this set up. The reactor and catalyst are doing their respective jobs, but a better
configuration is needed in order to vaporize the alcohol (without superheating the liquid), as well as a better set up to condense and separate the
product from the excess alcohol. The excess alcohol also needs to be automatically sent back to the vaporization unit. As such, this design mainly
serves to give others ideas; it is not a finished project intended to be used as a cookbook design.
A wooden dowel is covered with two-sided adhesive tape, and sandpaper is wrapped around the dowel one time (80 grit is used instead of 320 grit; 320
grit is unnecessarily slow):
  
The finished dowel with 80 grit sandpaper is used to sand a groove into the fire-bricks (2300F type), pausing to vacuum the dust from time to time.
Afterwards, Kanthal resistance wire is wound tightly around the same dowel.
  
The wire loosens up once tension is removed from it. The coil is grasped on either end, and carefully stretched out to the correct length. The coil
will be a little loose on the dowel, and will not fit into the fire-brick. To remedy this problem, one end of the coil is taped to the dowel, and the
other end is held loosely to the dowel with one hand, while spinning the dowel with the other hand. This easily tightens the coil around the dowel,
and it can now be fit into the fire-brick. Once the coil is untaped from the dowel, it will expand out slightly in the fire-brick, unspinning a few
times. Space out the turns a little if this is necessary. Finally, two more fire-bricks are placed on top, leaving the wire ends hanging out from
in between the bricks.
  
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Copper sulphate pentahydrate and magnesium sulphate heptahydrate (Epsom Salts) have almost the same molar mass. Prepare a supported copper catalyst
by weighing out 5 parts of copper sulphate pentahydrate to 1 part of magnesium sulphate heptahydrate by weight. Dissolve this in cold water with
heavy stirring, and precipitate the hydroxides with a stoichiometric amount of sodium hydroxide. Filter and wash the blueish precipitate on a
Buchner funnel with vacuum. Don't allow the precipitate to dry fully. While still damp, form small beads with the precipitate. Thread the beads on
thin copper buss wire like a necklace, leaving small loops in between each bead to space them out. When dry, the beads will shrink roughly 50%. The
dried beads should be about 70-80% the inside diameter of the reactor. Experience (and a mold) will help determine the correct size of the wet beads.
 
The following set up was not very effective in actual operation, but photos will be posted to document the process.
  
With a couple of hundred watts input to the reactor coil, temperatures in the tube averaged about 300C. This is about the temperature needed for
alcohol dehydrogenation with this catalyst, while also allowing for long catalyst life. Higher temperatures work better, but the catalyst sinters,
becoming ineffective as its surface area decreases. The finished "stack", as well as the reduced (and re-oxidized by room air) catalyst is shown.
 
[Edited on 11-4-2014 by WGTR]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I like the way you built the coiled furnace.
You say the beads don't work too well. Have you thought about making a catalyst bed out of a highly porous inert material like pumice or other
volcanic rock? I have such a support that I made from pulverized lava rock for barbeques. My pieces are ~ 1/8" in diameter. I soaked them in
concentrated H3PO4 and they are now drying in a desiccator. My intent is to use them to produce dry formaldehyde gas from (HCH=O)3. It's a project
that's been on hold for some time. My intent is to use the dry formaldehyde to make primary alcohols via a Grignard reaction.
[Edited on 4-11-2014 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Oxirane
Hazard to Self
 
Posts: 92
Registered: 19-9-2014
Member Is Offline
Mood: No Mood
|
|
Copper hydroxide seems to decompose at such low temp as 80C. How efficient it would be if I made a mixture of precipitated copper hydroxide in water
and stirred silica gel into it and filtered it so the hydroxide would accumulate to the granules and then dehydrate them by heating for proper copper
oxide?
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Thanks, I try to keep things easy if possible.
For some reason I misplaced the reference that I originally used, but here is one with similar information:
Attachment: Effects of Alkaline-Earth Oxide Additives on Silica-Supported Copper Catalysts in Ethanol Dehydrogenation.pdf (126kB)
This file has been downloaded 1023 times
The catalyst works fine as far as I can tell. The beads are porous enough that the gasses permeate them easily. When removing freshly reduced
catalyst beads from the reactor, they have a dark violet appearance. Cutting them open reveals bright, soft, copper. Problems that I haven't gotten
around to solving yet:
1. Vaporizing the alcohol without a carrier gas. I have saturated nitrogen with alcohol vapors in previous tests, and this worked OK. The problem is
that I have all the liquid nitrogen a chemist could want, but most other people do not. For this reason I want to keep the vapor phase 100% alcohol.
Conversion is lower this way, but the operation is simpler, and the unreacted alcohol can be sent back to the vaporizer.
2. Alcohol likes to condense on things. The boiling liquid alcohol in the vaporizer needs to be the coldest thing in the entire system, except for
the output condenser and return line. Under no circumstances can condensed alcohol be allowed to bubble into the reactor inlet, our drip back into
the catalyst bed on the outlet. If liquid makes contact with hot catalyst, the catalyst disintegrates. Vaporization needs to be smooth, without
liquid alcohol superheating and bumping. The most straightforward way of maintaining a healthy vapor phase would probably be to enclose the entire
system in an oven. The catalyst would run at a higher temperature dictated by its own heating element.
Methanol dehydrogenation is easier than that of ethanol. In fact, the above-mentioned catalyst is too active. Much of the formaldehyde will
decompose into hydrogen and CO.
Sabatier wrote a great deal on this. I was so impressed with the book that I bought a (new) copy:
http://books.google.com/books?id=qOjQAAAAMAAJ&pg=PA232&a...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Oxirane  | For most private chemists(or maybe in this context, cooks), nitroethane would be something that was more likely held behind locked doors than those
the hazardous substances legislation generally requires. Using nitroethane for acetaldehyde sounds something like making benzoic acid from
benzaldehyde.
|
cheddite,isn't nitroethane sold as dragster fuel.also there is a very good thread on making EtNO2 incase you cant buy it
oxirane ,i didnt understand your comment,are you saying possessing nitroethane is illegal,because it is not on DEA watchlist http://en.wikipedia.org/wiki/DEA_list_of_chemicals
[Edited on 5-11-2014 by CuReUS]
|
|
|
Oxirane
Hazard to Self
 
Posts: 92
Registered: 19-9-2014
Member Is Offline
Mood: No Mood
|
|
I mean that nitroethane is considered a niche product comparable to nitroMethane for most but cookery chemists, who consider it as a valuable
precursor. Nothing more to add on this subject.
Quote: Originally posted by WGTR  | Methanol dehydrogenation is easier than that of ethanol. In fact, the above-mentioned catalyst is too active. Much of the formaldehyde will
decompose into hydrogen and CO.
|
Do you mean that if my catalyst bed is too large, it will decompose most of formaldehyde?
I understand the linked paper as following:
- First in solution are dissolved nitrates of Mg, Ca, Sr and Cu, then mixed in Silica gel, then stirred, filtered, dried and calcined.
- Then nitrate of barium is dissolved ontop of the all previous, and stirred, filtered and finally calcined all over.
-Then the catalyst is flushed with hydrogen for 2 hours at 300C to produce active catalyst.
How can calcium, strontium and other be calcined at 450C from their nitrates while their decomp temperatures are way over 600C? Both temps are
somewhat easy to achieve with normal gas burner with insulated container, but that would bring some low-decomposing oxides, like copper, into such
high temperature, could we be afraid of heating them "dead", as some mentioned CaO can be done?
Is the strontium necessary, or should it work with Ca+Cu+Mg+Ba only?
How should the catalyst be protected from air, or should it be prepared in situ for each dehydrogenation reaction? Once the cats are applied on the
SiO2 substrate, it don't have to be done again anymore, but most likely all the metals will be coated by an oxide layer the second they meet the
atmosphere.
Should one use pure copper only for formaldehyde dehydrogenation if the reactivity is too large for previous metals?
It has been demonstrated that pure copper tube heated to dull red was capable of dehydrogenating ethanol into acetaldehyde in Versuchschemie forum.
They got from several hundred ml's of ethanol a 100ml of acetaldehyde. They never tested how long would the catalyst live.
[Edited on 6-11-2014 by Oxirane]
|
|
|
| Pages:
1
..
11
12
13
14
15
..
20 |