Portability
Harmless

Posts: 5
Registered: 2-10-2014
Member Is Offline
Mood: No Mood
|
|
Catalytic Convertor... for your lungs? Cobalt technology allows underwater breathing without an oxygen tank!
http://mashable.com/2014/10/03/crystal-breathe-underwater/
I've always known about how cobalt can adsorb oxygen into a reactive species.... but this is an ingenious application of it. Not unlike compounds that
can absorb and store hydrogen and then later release it with the right applied energy...
| Quote: |
Cobalt gives the new material precisely the molecular and electronic structure that enables it to absorb oxygen from its surroundings," McKenzie said.
"Small amounts of metals are essential for the absorption of oxygen, so actually it is not entirely surprising to see this effect in our new
material," she said.
The material, like a sponge, can absorb oxygen and release it many times over. Once the oxygen is absorbed it can be released with a small amount of
heat or by exposing it to low oxygen pressure, like a vacuum. The researchers are also investigating whether the oxygen release could be triggered by
light.
"When the substance is saturated with oxygen, it can be compared to an oxygen tank containing pure oxygen under pressure — the difference is that
this material can hold three times as much oxygen," McKenzie said.
|
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
While this may seem initially like a good idea, there are a lot of problems with it. Firstly, oxygen solubility in water is quite low - about 8mg/L
for seawater at 20°. This means you will have to process many many litres of water for each breath if you want to extract the oxygen from seawater,
so you will have to have some kind of pump.
Secondly, oxygen is toxic. Breathing 100% oxygen is ultimately fatal, so it needs to be diluted with another gas like nitrogen. Where are you going to
get that gas from?
Although the article doesn't say as much, I wouldn't be at all surprised if this complex is soluble in water, which raises the question of how you are
going to transfer the oxygen from the water to the complex.
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
This is a better source: http://www.sdu.dk/en/Om_SDU/Fakulteterne/Naturvidenskab/Nyhe...
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Looking at some of the claims for this Oxygen storage technology- Stores less O2 per volume or weight than Sodium chlorate. If storage/release
mechanism is not energy intensive, and occurs quickly enough, without requiring excessively high temperature swings- Could be useful.
What happens when one sticks a blasting cap into this highly oxygenated organic compound...
[Edited on 6-10-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
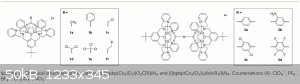
The article is Chem. Sci., 2014, 5, 4017-4025 (DOI: 10.1039/C4SC01636J).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Fulmen
International Hazard
    
Posts: 1726
Registered: 24-9-2005
Member Is Online
Mood: Bored
|
|
That's not quite as bad as one might think, but still a factor 30 less than air. IIRC a person uses about 500liters of O2 per day, this
equates into roughly 8mg/sec, meaning you'd need to circulate 1l/sec assuming 100% efficiency. That's going to make portable equipment impractical,
but given enough power it could theoretically be used for permanent installations or large subs.
As for diluting the O2, since the dilutant won't be consumed it shouldn't be hard to carry enough for extended periods of submersion.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Reading through this thread fish are pretty efficient at salvaging O2 from water. It would be useful to make a mechanism similar to fish breathing
system.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Random  | | Reading through this thread fish are pretty efficient at salvaging O2 from water. It would be useful to make a mechanism similar to fish breathing
system. |
I don't know about you but I would feel pretty nervous rerouting my circulatory system through an external set of gills. Sounds like it would work on
similar principles to a kidney dialysis machine.
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Submarines already have Oxygen generators and CO2 scrubbers that can provide breathable air for over 100 crew for months at a time. This
doesn't seem to be a big improvment.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by Oscilllator  | Quote: Originally posted by Random  | | Reading through this thread fish are pretty efficient at salvaging O2 from water. It would be useful to make a mechanism similar to fish breathing
system. |
I don't know about you but I would feel pretty nervous rerouting my circulatory system through an external set of gills. Sounds like it would work on
similar principles to a kidney dialysis machine. |
Yeah, well it could probabpy be possible but you would have to connect your blood vessels with the instrument and if it fails, well you are pretty
much dead.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Quote: Originally posted by Fulmen  | .
As for diluting the O2, since the dilutant won't be consumed it shouldn't be hard to carry enough for extended periods of submersion.
|
True, but that will prove to be much easier said than done. The dilutant (helium probably) will have to be constantly scrubbed of carbon dioxide,
which in concentrations above ~3% causes suffocation regardless of oxygen concentration.
On the fish topic, their oxygen isolation methods need not be as efficient as for humans, as fish are cold-blooded and thus use far less oxygen per
unit mass than us.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
This could be a real boon for breathing disability patients. A lot of interesting development is going to take place.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|