| Pages:
1
2 |
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Dissolving Neodymium Magnet in Citric Acid?
Hello everyone!
I've got some neodymium magnets where the nickel plating is coming off.
I'm wondering if citric acid would dissolve out the neodymium. I know citrates are mostly insoluble, but I've seen the abstracts of a few papers about
complexes formed by the Nd+3 ion that are soluble.
I'm just doing this for fun, and am wondering if any reaction would occur.
Thanks!
-cpman
[Edited on 9-21-2014 by cpman]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I may have achieved separation of terbium and iron in that manner. I dissolved a small piece of terbium metal and iron powder in citric acid
solution. After a day a tiny amount of yellow powder precipitated, leaving a clear solution. Try a small piece and tell me if you get a solution that
turns purple or pinkish in sunlight.
Otherwise use nitric or hydrochloric acid. Make sure you strip off some of the nickel plating, though.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
It does work (I think).
I just added the magnet to 5ml of 4 molal solution of citric acid, and there are little bubbles coming off of the parts of the magnet without the
plating.
I don't know if it is dissolving the iron or not, but I'll see how much of the magnet dissolves.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I think the ferrous citrate formed will precipitate from solution, while the ferric citrate will remain partially dissolved in the water.
If you achieve separation I'm sure MrHomeScientist and blogfast25 will be interested.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
It will take a while before I know. The H2 gas evolution is about the same speed as the gas evolution from zinc in store-bought white
vinegar.
Fortunately, this magnet appears to be one of the bonded powder magnets, which should increase the surface area of the Nd2Fe14B
alloy somewhat.
EDIT: I do not think very much, if any iron will dissolve, as I've put a saturated solution of citric acid on some iron. There is no evolution of gas
that I can see, which leads me to believe that the majority of the gas evolution in the solution with the magnet is due to the neodymium.
[Edited on 9-22-2014 by cpman]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
That would certainly be pretty amazing if citric acid dissolves only the Nd. I'm a little skeptical, but we'll see. Let us know if the solution turns
pink or purple, and changes color when viewing under fluorescent vs incandescent bulbs. You'll have to do some tests for iron in solution as well.
It's been a hell of an ordeal removing all the iron contamination from my magnet solutions, so I'd be very interested in any alternatives.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Does anyone know what the color of ferric citrate is? I got a yellow powder to precipitate from dissolving terbium and iron in citric acid. I'm not
exactly sure what it was. I know hydrated ferrous citrate is either white or reddish-brown (source, but I'm not sure if ferric citrate is soluble in water (ammonium ferric citrate is soluble).
The formation of potential bis(citrato)ferrate, bis(citrato)neodymate, and tris(citrato)neodymate are all possible and significant, especially because
lanthanides tend to complex well with O-donors, notably dipicolinic acid and water.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quite interesting, this use of citric acid.
I wonder if it would be more effective to use HCl (or HNO3) as solvent and then test separation ideas with citric acid separately.
[Edited on 23-9-2014 by blogfast25]
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
I'm only messing with citric acid because although I have 30% HCl (in the form of pool acid), it's contaminated with all sorts of crap, and I don't
want to have to distill HCl...
I know my citric acid is just pure citric acid. It's reagent grade.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
For what it's worth, Seaborg et al used Dowex-50 ion exchange resins and 0.2 M ammonium citrate/citric acid buffers to separate various actinide
elements. They often modelled their methods on experiences gained with REs.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
IIRC the standard procedure for separating individual lanthanides from their ores involves complexation with ammonium citrate or nitrilotriacetate.
The real question is whether ferric and ferrous citrate are soluble at all, and what their behavior is at different pH levels, as that may help remove
iron.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  |
The real question is whether ferric and ferrous citrate are soluble at all, and what their behavior is at different pH levels, as that may help remove
iron. |
This Sigma Aldrich page on ferric citrate links to a spec sheet (*.pdf):
http://www.sigmaaldrich.com/catalog/product/sigma/f3388?lang...
... that states solubility as 1 g/100 ml (hot water).
And acc. Wiki ferrous citrate is insoluble in water:
http://en.wikipedia.org/wiki/Iron(II)_citrate
[Edited on 24-9-2014 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Then I think we just might be onto something!
As I'm going to university in a week, one of my potential project ideas is to find efficient separation methods of iron and different lanthanides.
This may be applicable to recycling different types of rare-earth magnets that may be commingled when disposed of.
[Edited on 24.9.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Did you know citric acid is a good rust dissolver? Yellow to brown solutions are obtained, from personal experience.
Besides, why have none of the RE-freaks yet tried the chromatography?. I pondered to buy a column and try it, but I realised that making my own solid
phase (silica) would be much of a challange. Dan has posted the basics about chromatography here.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Citric acid is something I don't wanna introduce into my metal salts unless needed. Solutions will soon become moldy mess. Mold even grows on
concentrated copper citrate. Citric acid also forms complexes you can't really precipitate copper carbonate with sodium carbonate from copper citrate
solution. It forms a stable complex in a basic solution that is soluble. What then remains is to burn the evaporated messat high temp to get useful
salts again.
I don't recommend it to separate compounds unless really needed.
I like it to get rid of calcium salts from the solution for example though. Calcium citrate is surprisingly insoluble while some transition metal
citrates are still soluble. Interesting property.
[Edited on 24-9-2014 by Random]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Iron doesn't really form strong complexes from my experience, but based on the ease of producing ferrioxalate complexes, I wouldn't be surprised if
bis(citrato)ferrate complexes formed. Controlling the amount of citrate in solution may be the best way to prevent further dissolution of ferric ions.
I'll try RE chromatography ASAP when I get to university, Bezaleel.
And yes, Random, citric acid is a bacterial feast! I would suggest to crystallize the solution to prevent bacterial growth, as solutions are more
likely to be contaminated. Heating may be a good way to get either neodymium or iron to drop out of solution.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | | As I'm going to university in a week, one of my potential project ideas is to find efficient separation methods of iron and different lanthanides.
This may be applicable to recycling different types of rare-earth magnets that may be commingled when disposed of. |
Then you must contemplate the double sulphates (RE,K) method which for Nd works very well and appears to be used industrially for neomags.
Quote: Originally posted by Bezaleel  |
Besides, why have none of the RE-freaks yet tried the chromatography?. I pondered to buy a column and try it, but I realised that making my own solid
phase (silica) would be much of a challange. |
Dowex type resins work well too, so the solid phase isn’t the problem.
Building a column with sufficient capacity may be harder. And detecting the REs in dilute solutions may not be easy either: colour will only work at
fairly concentrated levels.
Yes, citrate containing solutions mould over very quickly, in my experience. Maybe a pinch of bactericide would do wonders?
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
The main issue with double sulfate method is the issue of other types of rare earth magnets being added to the mix. I want to ideally be able to
separate neodymium from samarium using some method.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | | The main issue with double sulfate method is the issue of other types of rare earth magnets being added to the mix. I want to ideally be able to
separate neodymium from samarium using some method. |
Considering the much higher solubility of Sm sulphate the method may well achieve that. I might run a quick test with my own samarium to that effect.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
I read that in the last 10 years or so it was discovered that Pr can be used as a partial replacement of Nd in RE magnets.
Such statements are found e.g. here
"Magnets: Praseodymium can be used as a substitute for neodymium in super magnets."
and here
"Dysprosium and Praseodymium are added as a replacement for some of the Neodymium to improve the corrosion resistance and to improve the Hci
(Intrinsic coercivity) of the "Neo".".
The latter site also has a table of a typical composition of a ND-magnet.
My guess is that up to 1% of Pr/Dy may be expected in Nd from magnets.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Brain&Force  | | The main issue with double sulfate method is the issue of other types of rare earth magnets being added to the mix. I want to ideally be able to
separate neodymium from samarium using some method. |
Considering the much higher solubility of Sm sulphate the method may well achieve that. I might run a quick test with my own samarium to that effect.
|
Are you sure about that? It seems to be the least soluble.
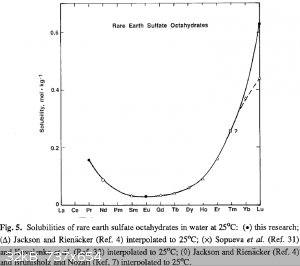
[Edited on 26.9.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ooopsie. Got my wires crossed, my bad. Ta for the reminder.
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Well, I now have a viscous, almost opaque brown-green solution.
I've taken a small amount (< 1ml) and I'm going to test for the presence of iron by adding zinc metal to the solution. First, I'm going to mix it
with some distilled water, then I'm going to neutralize any excess acid with sodium bicarbonate. Finally, I'm going to add solid zinc to the solution.
If I'm not mistaken, this should cause any iron present to reduce, while leaving the neodymium intact (if it even exists).
Does this sound like a good way to test for iron?
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
No, use thiocyanate if available. Or you can precipitate out some of the neodymium with sulfate.
But filter the solution first. And if possible post a pic - it'll help me more.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Now I'm quite confused. The solution mixed with distilled water that I neutralized with some NaHCO3 became much darker - almost opaque. I
then placed a cap on it, and it changed to a clear reddish solution. The weird thing is that it started out a light greenish yellow color!
I'm going to take some pictures and post them later today.
Also, the main solution has become VERY viscous - almost like honey.
I presume this is due to the bonding agent.
|
|
|
| Pages:
1
2 |