Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
isatoic anhydride synthesis
Isatoic Anhydride Synthesis
-------------------------
Introduction:
Isatoic anhydride is a very useful intermediate in industry for making things and stuff. These can range from pesticides to muscle relaxant
medications.
The following procedure starts from phathlic acid, undergoing thermal rearrangement to yield phthalic anhydride. Phthalic anhydride undergoes
hydrazinolysis with urea to phthalimide. Phthalimide undergoes a reaction with sodium hydroxide to form that alkaline salt, which is then reacted with
sodium hypochlorite and finally hydrochloric acid solution to make isatoic anhydride.
-------------------------
Procedure:
100g phthalic acid (or phathlic anhydride contaminated with the acid) is heated until molten in a 250ml beaker on a hotplate and held at this
temperature until the liquid goes clear, indicating loss of water and conversion to the anhydride.
This is poured out into a stainless steel pot and allowed to cool, before being crushed up and stored in a sealed container.
[It should be noted that Aluminium pie tins are better suited for this, as they can be deformed to help release the solidified disk of phthalic
anhydride.]
A 76g of phathlic anhydride yield results.
50g of phthalic anhydride is mixed coarsely with 10g of urea.
This is rendered molten inside a 1L flask, at which point effervescence occurs, building in rate until it fully foams up into an expanded cake
signalling completion of the reaction.
The flask is allowed to cool, and ~100ml demineralized water used to loosen the cake. The slurry is poured into a 500ml beaker and crushed with an
Stainless steel spatula before being vacuum filtered and washed with 500ml more water to remove traces of urea. This is spread onto aluminium foil on
a hotplate at 120C to dry. A 42g dry yield of phthalimide results.
In a 1L beaker, 42g phathlic acid, a stir bar and 100ml water are combined. To this is added 80g of ice, cubed or crushed, to lower the temperature.
Stirring is engaged on medium and maintained until the completion of reaction.
11.6g sodium hydroxide is dissolved in ~50ml water, or enough to get it fully dissolved. This is poured into the beaker with the phathlic acid. If
needed, more ice is added if previous ice has melted.
520g of 4.2% supermarket sodium hypochlorite bleach (unfortunately with detergents, avoid) is then poured in, ensuring melted ice is replaced. 4
minutes and 30 seconds later, 25g of ~33% Hydrochloric acid is added, and more ice added if needed. 4-8% NaOCl solution can be utilized, and the
weights scaled. The solution turns from clear straw coloured to a red hue, and a precipitate begins to form. 4 minutes later, 12g 33% HCl is added.
The reaction temperature is raised to 25-30C until ice has fully melted. Once at this temperature, the reaction is held there for 40 minutes,
resulting in a chocolate coloured precipitate in a reddish liquor.
The precipitate is vacuum filtered, and rinsed with ~3L water due to the detergents that were present in the bleach solution. Using plain sodium
hypochlorite solution would likely only need ~500ml to wash ions out, but solubility in water of the isatoic anhydride is not very significant. A damp
yield of 48g results.
The isatoic anhydride is dried at 60C on a hotplate in a glass dish. A melting point test is taken by placing a small sample of the isatoic anhydride
in a drilled hole on a small aluminium cylinder on a tripod with a thermometer inserted into another hole next to the sample of the same depth. This
is heated with a bunsen flame.
A melting point of 236 +/- 1C for the tan coloured isatoic anhydride is observed, as it melts and decomposes simultaneously. This is consistent with
internet search engine results.
----------------------
Discussion:
Some improvements noted. Not a very difficult reaction route.
Maybe someone can recommend a recrystallization solvent for the isatoic anhydride
Conclusion:
It works.
---------------------
References
"Conversion of Phthalic Acid to Phthalic Anhydride", Magpie, lab constructor
http://www.sciencemadness.org/talk/viewthread.php?tid=25566
--------
Synthesis of phthalimide, VOGEL'S Textbook of Practical Organic Chemistry (5:th edition), page 1065-1066
Phthalimide:
Method 1. Place 100 g (0.675 mol) of phthalic anhydride and 105 mL of concentrated ammonia solution in a 1-litre
round-bottomed flask fitted with a wide air condenser (10 mm in diameter). Heat on a sand bath, gradually at first until the mixture is in a state of
quiet fusion and forms a homogeneous melt (the temperature reaches 300°C in about 1.5-2 hours; all the water is evaporatedduring the first hour).
Shake the flask occasionally during the heating and push down any material whicj sublimes into the condenser with a glass rod. Pour the contents of
the flask while still hot into a porcelain basin, allow to cool and grind to a fine powder in a mortar.The phthalimide (95 g, 96%) is practically pure
and melts at 233-234°C. It may be recrystallised from EtOH, but the solubility is only slight (about 5%).Method 2. Intimately mix 99 g (0.67 mol)
of pure phthalic anhydride and 20 g (0.33 mol) of urea, and placethe mixture in a 1-litre, long-necked, round-bottomed flask. Heat the flask in an oil
bath at 130-135°C. When the contents have melte, effervescence commences and gradually increases in vigour; after 10-20 minutes, the mixture suddenly
froths up to about three times the original volume (this is accompanied by rise in temperature to 150-160°C) and becomes almost solid. Remove the
heat from beneath the bath and allow to cool. Add about 80 mL of water to disintegrate the solid in the flask, filter at the pump, wash with a little
water and then dry at 100°C.The yield of phthalimide, m.p. 233°C (i.e practically pure), is 86 g (87%). If desired, the phthalimide may be
recrystallised from 1200 mL of industrial spirit; the first crop consists of 34 g of m.p 234°C, but further quantities may be recovered from
mother-liquor.
---------
Isatoic anhydride synthesis, Fire_ass_lean
http://www.tandfonline.com/doi/abs/10.1080/00304948209354906...
http://www.google.com/patents/US4328339
"50 g (.34 mole) of finely powdered phthalimide was added to a 1 liter erlenmeyer flask and 125 ml of water was added along
with a stirbar. In a separate container, 14 g (.35 mole) of sodium hydroxide was added to a quantity of water sufficient to dissolve it. After the
hydroxide solution had dissolved and cooled somewhat, it was added to the phthalimide in the flask and magnetic stirring started. After a minute or
two, when almost all the phthalimide had dissolved, about 100 grams of crushed ice was added to the flask. Then, 295 g of 8.25% sodium hypochlorite
(.33 moles) was added substantially all at once under vigorous stirring. More ice was added as it melted to keep the solution cold. 4 minutes and 30
seconds after the bleach addition, approximately 3/4 of a total of 41 g (.35 mole) of 31.45% hydrochloric acid was added in one portion. Some white
precipitate was noted. Stirring was continued for another 4 minutes, then the remainder of the acid was added. The heat on the hotplate was turned on
to speed the melting of the ice and to bring the mixture to about 20-30C. At that point the heat was turned off and the mixture was stirred for
another 30 min. The mixture, which was a beige slurry with a slight pinkish tint, was suction filtered and washed several times with water. The cream
colored filter cake was somewhat water-logged and resembled tapioca. It was dumped out onto a pyrex dish and heated on low heat on the hotplate for 12
hours to dry. The product darkened somewhat during drying and became a light tan powdery solid. Total weight was 54.6 g (98.7% yield).--Yield seemed
somewhat high, perhaps because of residual moisture (it likes to hold on to its water and takes a long time to dry). OTOH, it could have just run
superbly smoothly.The product dissolved easily in alkaline solution but not in acid. However, it dissolved slowly in hot concentrated hydrochloric
acid with bubbling to give a clear solution that did not precipitate any solids on cooling (presumed hydrolysis to anthranilic acid with evolution of
CO2). On dropwise addition of aqueous ammonia, a flocculant white precipitate appeared (presumed to be anthranilic acid) which re-dissolved on
addition of sodium hydroxide solution. This may open up a new high yield route to anthranilic acid.This can be a tricky reaction to master. Timing is
essential. The interval between the addition of bleach and the addition of acid depends on temperature and can vary somewhat, but the optimum time
with icing seems to be around 5 minutes. In a clean reaction, the product is light colored and filters easily. It should not clog the filter
paper.There should be no foaming of the reaction mixture upon acidification. If there is foaming, it means that the acid was added too soon and not
all of the N-chloroamide has had time to rearrange. When this happens, the N-chloroamide hydrolyzes into phthalic acid, which precipitates and
contaminates the product, and noxious chlorine-containing gases are evolved which produce a thick head of foam.If too much time elapses between the
bleaching and acidification stages, side reactions will run rampant. The mixture will turn dark black and give a small yield of charcoal-colored crap.
"
       
[Edited on 8-9-2014 by Templar]
[Edited on 8-9-2014 by Templar]
[Edited on 8-9-2014 by Templar]
[Edited on 9-9-2014 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Hi!
Appears to be painstaking work, however if you include basic chemical formulae and equations in the begining it will add tremendous clarity to this
post.
Gsd
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Most of these reactions I have not had the time to research the mechanics for. Some do not have mechanics that I could find.
If there is anything I didnt specify, please ask. I think I was thorough in outlining all the reagents required.
Source material is also referenced.
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | 520g of 0.42% supermarket sodium hypochlorite bleach[/rquote]
Should be 4.2%, yes?
|
The less you bet, the more you lose when you win.
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Corrected, thanks.
Ref attached recommends use of acetic acid for recrystallization. Might be unnecessary, but I do not like having tan crystals... it suggests
impurity..
Probably xylene would work too.
Attachment: 386.pdf (151kB)
This file has been downloaded 779 times
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
Nice job! When you say you add the sodium hydroxide to the phthalic acid, you mean phthalimide, correct? If not, I am misunderstanding something
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Yes, another error. Phthalimide reacts to form the alkaline salt, then hit with bleach and then HCl.
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Perhaps you should add a reaction scheme to this post.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
US patent 3,324,119 have some experimental data of different factors leading to a change in yield. The main factor is hydrolysis of phthalimide before
hypochlorite is aded. I'd say it's possible to obtain the same intermediate without the need of high ph in the beggining of the reaction, but in this
case phthalimide has extremely low solubility, so we either need a solvent like dmso, or we can continue to stick NaOH-water, but keep the temperature
and the dissolution time as short as possible.
Also there's some info in the US 4,328,339 which is consistent with the above patent, and US 3,867,951 has some more information about influence of
hydrolysis on the yields and also some nice time frame descriptions.
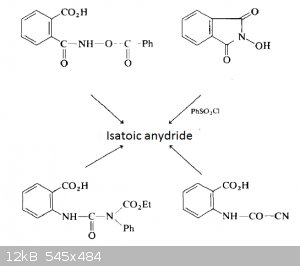
Picture from an article "Isatoic Anhydrides and Their Uses in Heterocyclic Synthesis", published in the "Heterocyclic Chemistry" Vol. 28 about some
possible direct precursors of isatoic anhydride.
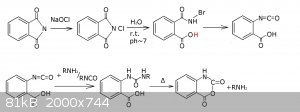
There's an overview of isocyanates properies and their characteristic reactions http://www.poliuretanos.com.br/Ingles/Chapter1/131Isocyanate...
So the reaction mechanism the way I see it after reading all this stuff:
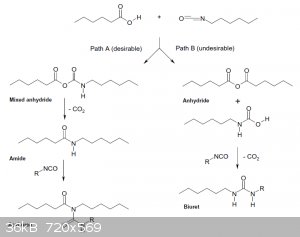
UPD: Okay, I've encountered an article saying that the initial reaction of carboxylic acid with isocyanate leads to a mixed anhydride. http://www.sciencedirect.com/science/article/pii/S0040403904...
And a patent telling the same US20130338247
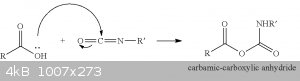
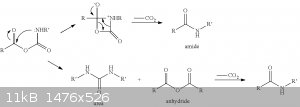
And even one more US20060167124

So there's no catalyst by amine (the last picture before the update most likely is not correct) but a direct conversion into carbamic-carboxylic
anhydride.
[Edited on 22-4-2015 by byko3y]
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
EDIT: lacking a decent purity analysis for this procedure I cannot vouch for what % purity the isatoic anhydride produced is. If the impurity is
primarily phthalimide, then a melting point test will not indicate much information in regards to purity, save for evidence of decomposition of the
isatoic anhydride. This should have been mentioned before but I had assumed that all phthalimide would hydrolyze to phthalic acid in the basic
conditions, which would have a clear effect on melting point.
Hey thanks for getting a rxn scheme for that. Quite interesting and very helpful.
I was wondering why there was so little literature regarding synthesis of therapeutic quinazolinones from isatoic anhydride. Maybe isatoic is more
expensive than the comparative starting blocks of anthranilic acid, acetic anhydride, and POCL3?
Or maybe the reaction yield sucks/doesnt work :/
But in theory it definitely should; the isatoic anhydride ring should be opened by an alkylamine or aniline type compound, then the inner ring closed
by use of an acetylating agent such as acetylacetone or acetic anhydride.
US 3,867,951 leads to a tobacco substitute. may want to double check that one
I think our two relevant patents are
http://www.freepatentsonline.com/3687951.pdf
http://www.google.com/patents/US4328339
This would work great, buuuut... phosgene
http://orgsyn.org/demo.aspx?prep=cv3p0488
So the real problem here is knowing the purity of your final isatoic anhydride. If it is contaminated with phthalimide, there will be no effective way
to tell because their melting points are within 5C of each other.
If contaminated with phthalic acid, there is about a 30C difference between this and isatoic anhydrides melting point, which would make it much easier
to see if your product is impure.
This is no doubt a fascinating but simultaneously difficult reaction.
An effective way to tell phthalimide and isatoic apart but chemical tests would be ideal, I will properly test the steps outlined in fire-ass-leans
procedure.
I have some anthranilic acid lying around, but it is the chloride salt and melts at 168C. Now thats it free of bird repellent veggie oils and
additives, I will try isolating it as the acetate salt once its been basified with NaOH, and I am hoping an acetylation of this acetate salt using
acetic anhydride will work.
It should, as acetylation by AA produces acetic acid, which presumably reacts with zwitterionic anthranilic acid to make the acetate.
[Edited on 22-4-2015 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Anthranilic acid has most of the chemical properties of isatoic anhydride. Don't let the name "anhydride" deceive you - it can barely bind a water, so
it's useless as anhydride.
Here's my attempt to detect phthalimide purity http://www.sciencemadness.org/talk/viewthread.php?tid=19662&... you can use it to detect the phthalimide content in the final product. And this
one phthalein and fluorescein tests) for sure http://jscienceclass.blogspot.com/2012/07/special-reactionst...
|
|
|
|