| Pages:
1
2
3
4 |
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Thulium (III) color
Hi all,
I recently obtain a small sample of Thulium metal. I broke off a small piece and added a few drops (maybe 5 mL) of 1M sulfuric acid. The metal fizzed
and dissolved. Based on what I have read in the Wikipedia article on Thulium, I thought that I would end up with a pale green solution but it was
clear instead. Thinking that maybe it was too dilute, I boiled off most of the water and a white precipitate formed. I added a few mL of distilled
water and the precipitate dissolved again, producing a clear solution. I then wondered whether the acidity of the solution, from residual H2SO4, might
be altering the color. So, I neutralized the acid with NaHCO3. When I got close to neutral I overshot slightly, and the solution became milky white
suddenly. I let it sit and it has formed a clear layer on top of a milky one.
Any idea what is going on - especially why I never noted any green color?
I've also heard that the Thulium III ion should fluoresce blue, but none of the references I've found say what to excite it with to produce this. I
tried 385nm UV on the clear solution and saw nothing.
Thanks,
Sean
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Here's what I can tell you:
a) Rare earth elements have REALLY weak f-shell transitions. This leads to REALLY narrow absorption bands and REALLY dramatic color changes in
different light sources. Try both sunlight and tube lighting. Chances are, the green color is light.
b) Thulium(III) fluorescence may not be activated by a longwave light - try a shortwave source if you have one. I have never worked with thulium
compounds, but terbium sulfate does not fluoresce under longwave UV light.
c) Thulium sulfate is not very soluble, but it is soluble. Keep that in mind, because you're working with a rare element.
Where'd you get your thulium?
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Thanks Brain&Force. I did look at it under both sunlight and regular incandescent. I don't have fluorescent lights in the room I use for chemistry
stuff. I do not have a shortwave UV source handy, although if I feel brave, I might try defeating the interlock on an old UV EPROM eraser I have so I
can hold the door open and expose the sample that way.
I got the Thulium as well as Erbium from an eBay seller called rareearthmetalsllc. I mainly want them for element samples but I am always a bit
suspicious of eBay purchases so I wanted to try a test to gain some confidence that it really is Thulium, and thought that the color change would be a
way of doing that.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
As much as I hate to dampen your hopes I firmly believe you were not sent Thulium. What price/quantity did you buy? This may give yet another clue
since we all know how rare/expensive it is. From the wiki page you mentioned : "Thulium dissolves readily in dilute sulfuric acid to form solutions
containing the pale green Tm(III) ions, which exist as [Tm(OH2)9]3+ complexes"
I think if it were really Thulium you would have seen the green color. Assuming your acid was not too concentrated (which from your post it sounds
like it was not), I see no possible way you would not have seen a green solution. I tried searching for that seller name with no luck. Either he does
not exist or you spelled it wrong. Doubtful as I also looked at every single ebay auction concerning Thulium and no name appeared remotely similar
with any spelling I saw. I have 5 gm which was not cheap I bought from either Hamric (IIRC Metalium now) or Emovendo. Been a few years so I don't
remember which one as I bought many items from both. In any case either can be trusted, SoCal as well. Provide a link to something this seller has on
ebay now as now I am curious about this seller.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Here is the seller, I have looked at some of his rare earths before. It's odd because he has no negative feedback and I think some other members
have purchased successfully from him before.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
sbreheny:
Sadly, I think you may have been had. No green colour at all, not even the hydroxide, is rather suspicious. Unfortunately there are no real quick and
easy tests to identify a REE (although colour of salts is a clue).
At the moment the seller does not advertise thulium. I think you may have to complain on the grounds of 'item does not match description'. Do check
that description first.
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This kind of things happens sometimes. At this moment, I have a similar experience with a seller, who sells ultrapure very fine vanadium powder (5N,
99.999%). Of course, I doubt such purity claims, but still, having 100 grams of pure vanadium powder for EUR 17 or so is very good. But I received a
bottle with 100 grams of dense grey powder, the label telling it contains vanadium powder, but on doing experiments with it, I found out it is just
zinc powder (gives colorless solutions with dil. HCl with production of H2, gives white precipitate with NaOH, which redissolves in further NaOH and
also dissolves in ammonia and gives white precipitate with dil. solution of Na2S). I contacted the seller, and he made excuses and told me he will
send the correct item to me. We'll see...
Just contact your seller, explain how you tested his material (I also explained to my seller all my findings with my experiments and also explained
what should have occured if it really were vanadium) and see how he reacts.
[Edited on 29-6-14 by woelen]
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Hmmm. Thanks for all the replies. I purchased 1.125g for $14.50. This is comparable to the price which Metallium charges.
Another possible clue is that this sample is very slightly attracted to a magnet. I can just barely make it slide along a glass surface with a strong
NeFeB magnet applied from below. This is consistent with the Wikipedia article's statement that it is paramagnetic at 300K, although I have not yet
found a reference to how strongly paramagnetic it is.
[Edited on 29-6-2014 by sbreheny]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Quote: Originally posted by sbreheny  | Hmmm. Thanks for all the replies. I purchased 1.125g for $14.50. This is comparable to the price which Metallium charges.
Another possible clue is that this sample is very slightly attracted to a magnet. I can just barely make it slide along a glass surface with a strong
NeFeB magnet applied from below. This is consistent with the Wikipedia article's statement that it is paramagnetic at 300K, although I have not yet
found a reference to how strongly paramagnetic it is.
[Edited on 29-6-2014 by sbreheny] |
Yup, that's kinda what thulium does. (skip to 7:03)
<iframe sandbox width="560" height="315" src="//www.youtube.com/embed/62dez4tD5Ok" frameborder="0" allowfullscreen></iframe>
It'll help if you do two things:
a) look at it under fluorescent (tube or CFL) lighting
b) take pictures! Thulium oxide tends to be a pretty faint green.

Best image I could find. It's not that green. Not even mint green.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Quote: Originally posted by Brain&Force  | | c) Thulium sulfate is not very soluble, but it is soluble. Keep that in mind, because you're working with a rare element. |
Thulium sulfate is very soluble! Rare earth sulfates show very different solubilities:
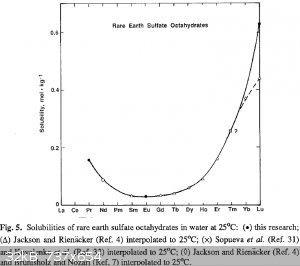
http://link.springer.com/article/10.1007%2FBF00651459#page-1
The rare earth hydroxides are not that deep in colour as the salts in my experience. Sm(OH)3 for example is nearly white, whereas the sulfate is
yellow in the crystalline form.
Here you can see 60 grams of Thulium sulfate octahydrate:

This is the true colour which you will see with your naked eyes in daylight. Thulium oxide is weaker in green colour, but still very slightly
greenish. At 405 nm none of the two compounds show a fluorescence.
[Edited on 29-6-2014 by Pok]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
"Another possible clue is that this sample is very slightly attracted to a magnet."
Along with a few other elements. However the lack of green tells something about electronic structure which I don't think I could overlook. Remember
the seller could be OK but they themselves could have been taken by who they buy from. Especially with little experience as a feedback of 181 implies.
This seller possibly has no way to test the elements he is selling, and equally possible is the thought that no one who bought Thulium from him did
the tests you discuss in this thread. Woelen said it, contact the person with your questions and concerns. I wouldn't rush to leave him negative
feedback as his score is a perfect 100 and one has to work hard to maintain this. As I said possibly he was taken as well but is willing to make it
right. Try making the oxide out of some and compare it to what is known concerning properties.
Just had another thought. What is the source of your acid? How pure is it? I ask as many use sources from OTC products which contain many impurities,
which could possibly radically alter the reactions you were trying to conduct. Something to consider. His Dy looks correct if the pictures are of the
actual item he is selling. I have a lot of Dy and it looks identical to his images in the listing. All in all the seller looks legit yet anyone can be
taken including him. A good seller will correct the issue if there is one so your next step should be talking to him.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
This post piqued my interest. I have a set of Lanthanides bough a few years ago when they were a bit cheaper including 1 gram of Thulium. I sacrificed
0.3 of this sample to investigate. It dissolved easily in 2.5ml of 1M sulphuric acid (Slight excess of acid used) to give a very pale
green solution. I did a visible spectrum of this which shows a typical lanthanide absorbance spectrum with some sharp peaks especially at about 680nm.
I have attached pictures of the solution and the spectrum.
 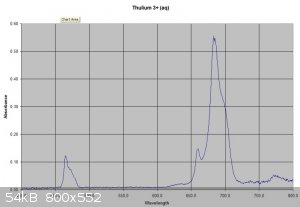
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
This reinforces my thought about his source of acid. I had been wondering if some other impurity/reaction could produce something which would tend to
visibly mask the green color sbreheny had been looking for. Especially after carefully looking at all the items for sale, all of which appear to be
legit. Is it possible some side reaction has 'covered up' the green?
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Iron, iron, iron.
In order to work with the lanthanides, you MUST have an iron-free source of acid. This is absolutely important, especially if you're going after the
fluorescence, or any color changes.
Here's the kicker: terbium sulfate is supposed to be white, but a tiny amount of iron (< 3%, I think) turned it slightly green. Not as green as
Pok's thulium sulfate, but still noticeably green, similar to the color of the thulium oxide I posted here.
And that's interesting, Pok. As expected, terbium sulfate was quite insoluble when I was working with it. I thought thulium sulfate would be the same.
Here is the spectrum of fluorescent lighting:

At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Thanks for all the help. This forum is great! The photo of the Thulium sulfate solution looks so pale green to me that I would be in doubt as to
whether I was actually perceiving green or just thinking it by auto-suggestion.
In my previous attempt, I used technical grade sulfuric acid. I also have some ACS reagent grade (began as 98% but I diluted it with distilled water).
I re-tried the reaction with the purer acid and I don't see much difference. But, I boiled off the water and I am now drying the precipitate to see
what it looks like when dry. It looks slightly brownish-yellowish right now, but mostly white.
Additional data points - the metal does react with water (I can see bubbles forming under a microscope), but very slowly. Even if I heat the water, I
didn't get much of the metal to dissolve. This could be due to the metal being a solid chunk and not porous enough to present adequate surface area.
Also, reaction with iodine is supposed to produce a yellow salt. I tried that, with enough heat to sublime the iodine, and I did get a yellow residue
around the edges of the chunk. Again, I think that it might need to be made into a powder to react fully with the iodine.
I tried filing a small piece to get a powder but it is actually quite hard and doesn't file easily.
When I get the precipitate dried I will take photos of it as well as the bulk material and post them.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Brownish-yellow? Try a thiocyanate test on your acid.
I was never really able to get bulk terbium to react with iodine with simple, but powder should work. If it still doesn't work, add a few drops of
water to the mix.
Also, try burning a small piece and figure out what color it burns. It'll probably be greenish:
<iframe sandbox width="560" height="315" src="//www.youtube.com/embed/sPd-sJ8ktRM" frameborder="0" allowfullscreen></iframe>
[Edited on 29.6.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
some time ago I read an article about thermoluminescense and it gave thulium oxide as one of the examples, it apparently glows red well below "red
heat" c200-250 C. As far as I can ascertain this is unique amongst the RE's. Europium oxide itself and europium or samarium produce red and orange
fluorescence in some compounds ( eg calcium tungstate) but as far as I can see from the information I have found their oxides are not
thermoluminescent. However, thermoluminescense is usually a response to past irradiation so it may need to be irradiated first (with shortwave UV?).
I don't recall where the article was from but it shouldn't be too difficult to tract it down. Lots of papers about thulium and dysprosium induced
thermoluminescense in other substances come up on a google search. Particularly in calcium sulphate which should be fairly easy to produce.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
"I tried filing a small piece to get a powder but it is actually quite hard and doesn't file easily."
Reminds me of a thread around here somewhere and the troubles I had making glow powders. I had to give up hack sawing my Lanthanides since Fe in
incredibly small amounts poisoned my work killing the glow. The statement by Brain&Force "Iron, iron, iron" is something to think about. I was
mostly working with Eu, La, Nd as dopants for both Sr and Ca Aluminates. I resorted to using abrasive cutting wheels. This trouble was created by Fe
in such tiny amounts I could not even determine it was present. Hack sawing a 2 pound block of Eu and the putting the unused portions back in the
paint can of oil left microscopic Fe particles in the oil which contaminated the Lanthanides. As I removed a piece the Fe particles stuck to it,
carried in the oil. I washed my pieces with Acetone many times putting them back into new cans with new oil. Only then did my contamination troubles
cease. A file will also cause this trouble. I think you should use a Dremel with an abrasive cutting wheel to cut it. Not sure what would work to
yield Fe free powder for you but a Ball Mill with ceramic media might do it.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|

Where can I get one? Can I get one for free? Please? 
[Edited on 29.6.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | some time ago I read an article about thermoluminescense and it gave thulium oxide as one of the examples, it apparently glows red well below "red
heat" c200-250 C. As far as I can ascertain this is unique amongst the RE's. |
I also read about this property and can confirm it. But I think the temperature is much higher than 200-250 °C. Tm2O3 glows "carmine red" when heated
directly (!) with a bunsen burner flame. Unfortunately, the colour can't be catched with a camera. If heated to higher temperatures, the colour turns
to yellow. Both colours are clearly different from a usual thermal radiation.
"The oxalate was of a very pale green tint when moist. Artificial light increased this tint so that it became a delicate pale green color. The
oxide, obtained by igniting this oxalate, also showed this green tint and yielded a carmine glow, when heated carefully by a Bunsen burner."
http://pubs.acs.org/doi/abs/10.1021/ja02221a007
Or here.
[Edited on 29-6-2014 by Pok]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
China but not cheap. In fact had I not bought my Lanthanides about a decade ago no way could I do it today. Some like Nd (2 lb) and La (1.5 lb) I
bought from Hamric around 2004 when he had a killer deal for these two large ingots. Today under his Metallium name he has La 50 gm for $38 and Nd 50
gm for $45. Meaning he is paying a hell of a lot more for everything today as opposed to a decade ago since I doubt in experimenter quantities any
sellers are more reasonable and fair in pricing. Emovendo is similar but I think he is more into selling magnets than elements today.
I've seen prices go insane in the last 5 years. To be honest I do not see why either. Eu is going up to $40/gm on ebay in sample quantities looking at
several sellers. No way could I start out today and it does not look like this trend is ever going to stop. Look at Gold. $271/oz in 2001 and
~$1315/oz today.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Considering that europium is more common than iodine in Earth's crust, I don't see how it can be so expensive. And demand is pretty low anyway.
Samarium, however, is much easier to obtain. Emovendo doesn't sell any rare earths now. I know of no other suppliers.
Once the US gets its rare earth mines back online prices will (hopefully) drop dramatically.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
That demand is low is another reason why the prices are high, rather contradictorily to supply and demand theory. Since there is no reason to develop
more efficient means of mass production to meet a high demand, most rare earths are highly priced partly due to the effort it takes to process them.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Mostly finished drying the precipitate. I think that the slightly yellowish-brown color was due to concentrated sulfuric acid - the drying removed the
water but not the acid until it reached the azeotropic concentration. Then, I increased the temperature of the hotplate to boil off the azeotropic
acid (only a few drops). Note to self: it only takes a few drops of nearly pure h2so4 to produce a lot of irritant vapor! I had a fair amount of
ventilation but not enough - I had to put on my respirator to go back into the room to open the window wider and put a fan in the window. It's only
slightly detectable now.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
OK, here are the photos.
Crystals on Watchglass
Crystals magnified
Raw Metal
|
|
|
| Pages:
1
2
3
4 |