numos
Hazard to Others
  
Posts: 270
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Aquisition of Phenol?
Where do all of you get your Phenol? Buying it is out of the question, unless there is some OTC source I am unaware of, but I've looked online and
apart from the usual Sigma and Aesar, nothing stood out.
... and according to Wikipedia "Phenol is so inexpensive that it attracts many small-scale uses." ... so inexpensive. ... right
I realize I can make it from Toluene, but granted that I live in California, "the golden state where everything causes cancer" getting Toluene is
bound to be harder than Phenol itself.
Aromatic compounds are difficult to get in general here. Any input is appreciated.
Thanks, numos.
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
Its synthesis from salicylic acid has already been described in the forum before...
http://www.sciencemadness.org/talk/viewthread.php?tid=27617
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
What do you intend to do with the phenol? I've been doing organic chemistry experiments for some time and I find that phenol is far less useful than I
had imagined, barring a few specific syntheses. Other starting materials are often better choices. The big issue is that phenols are a synthetic
dead-end. You can make ethers and esters from them, but it is extremely difficult, borderline impossible, to make them into any other functional
group. There are very few reactions that require phenols to modify the benzene ring further.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
numos
Hazard to Others
  
Posts: 270
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Good question... I don't know. I have no specific purpose for it, however as you stated, esters. But then isn't that the love of chemistry? Some
people here work in a chemistry field, others just enjoy the acquisition of pure substances.
...I still plan to work as a chemist though.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
First start with this:
http://www.valleyvet.com/ct_detail.html?pgguid=7e9ef644-64d6...
Read this:
http://www.sciencemadness.org/talk/viewthread.php?tid=23930
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
I have another suggestion as start point. You can start with wintergreen oil (almost pure methyl salicylate (up to 99%), easily found in pharmacies,
cheap at walmart), mix it with a solution of NaOH and heat it for around 20 min if I remember. This gives sodium salicylate. Simply acidify and you
get salicylic acid with a possible yield of around 90% +. Extremely easy and cheap. I did it and caracterised it and it worked like a charm according
to IR spectrum and melting point. You can find a protocol online for the details.
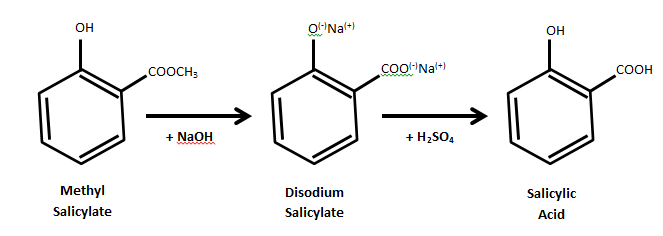
[Edited on 6-6-2014 by alexleyenda]
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
if you lived in Europe I could give you a source that supplies it for 15eur/kg, but in the US things are so difficult to get.
Phenol smells awesome, I keep a sample in my room just to smell every now and again. It somehow smells pure, the same way mint or lemon does. I think
it has something to do with an early childhood memory.
all above information is intellectual property of Pyro.  |
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
Haha, I like such statements, you know, things everyone does :p
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
also good for the occasional execution  my version of a home defense weapon my version of a home defense weapon 
all above information is intellectual property of Pyro.  |
|
|
numos
Hazard to Others
  
Posts: 270
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
I'm not quite sure I follow... Are you going to run at them brandishing a syringe? I see a few possible faults with that plan. 
Still, I'll probably end up using the Salicylic acid approach sometime in the near future.
|
|
|
EdMeese
Harmless

Posts: 16
Registered: 25-1-2013
Member Is Offline
Mood: No Mood
|
|
uh .... Ebay? http://www.ebay.com/itm/35-grams-PHENOL-Technical-Grade-/130...
That's pretty far from 15$/kg, but ... buy in bulk n save n all
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Oxford ChemServe on ebay for phenol on this side of the pond ─ and he's (hiding his light under a bushel) one of us . . .
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
numos: Yes. i'l run after them brandishing a syringe saying: ''stop! hands up! schnell!''
all above information is intellectual property of Pyro.  |
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
[Edited on 11-6-2014 by HeYBrO]
|
|
|