| Pages:
1
..
9
10
11
12
13
..
33 |
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | And the cave man said, Smack! Let there be fire! Fire in the hole! It's magic! Now we're cooking.
If a little nitroglycerin was soaked into that ball of tinder ......kaboom.
|
It is amazing indeed. When I first became introduced to some of these primitive technologies I think I felt a little bit of what the cavemen felt when
they first discovered some of these things. I have a science background and it is still quite amazing.
Yeah, I have been making jokes about putting a little nitro in one of those things for a while. Of course you could seriously hurt someone that way
with even a tiny amount. Gets people's attention when you suggest it though, that's for sure. 
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
fludyoptasyphos
Harmless

Posts: 37
Registered: 1-4-2010
Member Is Offline
Mood: No Mood
|
|
gerald l hurst said ''BTW, Astrolites A and G were pussycats compared to some of the other
letters that nobody ever wrote about in public''
some one competent can help me ? i need to know what kind of molecules he talk about (triazoles tetrazoles etc furazan oxan etc fefo formals other ,
else , idk, help)
✖ reaktiv ✖
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2.0g of Picric Acid Initiated with 0.20g of DDNP & 0.05g of Lead Azide (Unreinforced Configuration)
This test was the same as the last except that about half the loading pressure was used for the DDNP primary and the lead azide primary. Approximately
20MPa of pressure was used to press the primaries.
The results of this test were very similar to the last. However, on close examination of the witness plate it was clear that a thicker scab was blown
off in the last test. It was estimated that the scab removed in the last test was approximately 50% thicker. This was, however, a crude approximation
based on observation and it would have been difficult to measure accurately.
The holes on the right, in the witness plate shots, are from this test.
  
 
[Edited on 24-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That is likely velocity loss for the base charge. To test for the possible use of a lowered amount of DDNP, the suggestion would be to load 1.5 grams
of the base at the previous pressure and then a 0.5g increment at the lower pressure
associated with the DDNP / Pb Azide. That should get the performance back about where it was or possibly better. Dropping the DDNP to .15g in that
progressive loaded configuration would tell the story on whether such tuning has any benefit or not. If the performance is restored, then at the .2g
loading you will have gained headroom above the minimum. Once you know where the transition point is for a particular loading then you know how much
"extra" you are allowing with an arbitrary selected loading above that minimum that assures reliability. Progressive loading for the base is a
conventional loading method to provide an easier initiated intermediate so the amount of initiator required can be reduced, or for providing more
reliability with less lost velocity for the main part of the base charge.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2.0g of Picric Acid Initiated with 0.20g of DDNP & 0.05g of Lead Azide (Unreinforced Configuration)
This test was the same as the last except that the last 0.5g of the 2g picric acid base charge was pressed at approximately 20MPa, or about half the
pressure used to press the bottom 1.5g. As in the last test, the DDNP primary and lead azide primary were pressed at the lower 20MPa pressure.
There was noticeable improvement in comparison to the last test. A clean hole was not blown through, but there was a hole torn through and the scab
removed from the back of the plate was similar in thickness to the one removed in the second last test where the base charge and primaries were
pressed to higher density.
A different cap casing, as suggested by Rosco, could improve performance. The picric acid I am using is over 98% pure, as determined by melting point,
but my melting point apparatus is not accurate enough to be able to determine if the sample is closer to 98% pure or 100% pure. The cap diameter could
likely be reduced from 7.6mm. The Chinese primary explosives presentation, discussed earlier in this thread, specified 6mm casings for DDNP (but also
a reinforced configuration). The casings could, for instance, be reduced to 6.5mm. This would reduce the primary explosive weight needed and would be
a large enough diameter for the picric acid base charge to still function properly. There are likely other small factors that could possibly be
changed which could reduce the minimum primary explosive weight needed.
The holes on the right, in the witness plate shots, are from this test.
  
[Edited on 24-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yeah that is tracking with what I was expecting could happen. I wouldn't recommend going to the lower diameter with just using DDNP alone as the
initiator. But a mixture of the DDNP with silver azide or lead azide or a tandem arrangement could work at the lower diameter but still that pushes
the minimum for the base charge so the increased diameter like you are using is likely better for reliability and performance. A more sensitive base
charge would be better for a lower diameter. The progressive loading could be tuned further if you were using a metallic press pin and a separable
die to hold the capsule, you could go up to 8,000 p.s.i. on the first increment of base charge. The increments can be stacked at stepwise decreasing
loading pressure, as a progressive loading.
The same method translates to other configurations generally no matter what material base charge is being used. And that would be the technique used
if the entire charge was a single component DDNP loading, the lower increments would be effectively dead pressed but transitioning gradually to
regions that are not so that the detonation wave would initiate easily there in the lower density material and then accellerate through the increasing
density regions to maximize the velocity. The method is used as general practice even for base charges of PETN or RDX or anything else in either
compound detonators or single charge material caps.
Based on the plates though, I would say your idea about the including of the azide with the DDNP changes the initiation behavior of the DDNP since the
earlier tests at the higher compression are showing better plates. For DDNP the azide in tandem is drastically changing things as you supposed and
the higher compression is improving the performance, since the DDNP is functioning as a sensitive intermediate base charge initiated by azide, it
doesn't exhibit any dead pressing effect since it isn't operating in a DDT mode at all. It could be that the larger diameter is changing things also
from what the data chart is showing for what was likely a smaller diameter test and that data is not translating as would be expected. This same
synergistic effect happens with lead styphnate in tandem or mixture with azide where the usual DDT mode for the styphnate is skipped entirely and it
goes high order simultaneously with the azide, as a sympathetic detonating mixture. DDT like Elvis has left the building when azide is mixed in with
the usually DDT mode material. Basically these two tests confirm your original premise and illustrate how the data charted in the literature can be
in part but not completely translatable to predict what will occur in a different configuration. Obviously the charts would likely apply to the DDNP
used alone but not to the synergistic effect gained by using lead azide as the primary initiator but having the DDNP function as a coupling charge
operating as a sensitive intermediate base charge.
Adding that complexity to the configuration the data charted for the single component DDNP no longer applies. A similar effect will likely occur with
silver azide or other unequivocal initiators used to greatly enhance accelleration of DDNP from leisurely and sluggish DDT mode to a more
instantaneous high order detonation which makes available its potential energy immediately instead of as a delayed kind of gradually kindled and
building effect as would come from a DDT.
Generally, this is a similar principle as applies to azo-clathrates and double salts where an unequivocal initiator is made a conjoined twin with a
DDT material to create a synergistic hybrid. There is likely a lead azide / lead pentanitronaphtole double salt or basic lead complex clathrate
possible for such a scheme also as one of my "unpublished hypotheticals" now no longer a secret private speculation.
http://www.sciencemadness.org/talk/viewthread.php?tid=389&am...
There are other nitrated phenolic compounds whose lead salts exceed in brisance lead styphnate in high order mode and would probably form clathrates
or double salts with lead azide that could have interesting synergistic properties and would be more powerful than some already identified compounds.
These would likely be interesting candidates for experimentation, even though the novelty of "green" technology seems to be the current interest.
Existence of such materials has been mentioned in general terms in the literature a long time ago, but without any specific examples being given.
Sometimes it may be that such materials were known but were never investigated or published and simply became lost art.
[Edited on 24-5-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the good ideas. I have seen the progressive loading pressure idea in a patent or something before, but I never really applied it before
except for lately with these DDNP tests. Of course there are different levels of understanding. When I first saw the concept in a patent, my
understanding and ability to apply the concept was much less than it is now.
I just had a thought that a possible improvement could be a bushing to reduce the diameter (increase the thickness) of the lead azide top charge, or
increased lead azide top charge weight, but after consulting the attached table from the Matyas text it became clear that the 0.05g of lead azide
pressed like it was in a 7.6mm casing was actually just about the right amount to give a slab thickness producing maximum or near maximum lead azide
detonation velocity.
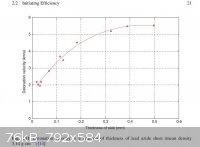
[Edited on 24-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Miznay-Schardin effect for platter charges is what is desirable for an initiator pellet, where there is a ratio of thickness to diameter that results
in function as a platter charge which is a type of ribbon charge having directed force enhancement as compared with just a spherical bubble force
effect. The effect occurs at about a 1:3 ratio IIRC. Claymore mines and shaped charges exploit the effect.
http://en.wikipedia.org/wiki/Misznay%E2%80%93Schardin_effect
[Edited on 24-5-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have seen references that state that it is hard to take full advantage of many of the shaped charge effects in a very small charge size (as in a
blasting cap). I don't have a lot of experience with this so I personally couldn't say for sure one way or the other. It is interesting to see how
much efficiency we can wring out of the DDNP and azide, but practically speaking when charges are this small it probably makes more sense to just add
another 0.2 or 0.3g of primary just to ensure high performance. Experimenting with the limiting case is very useful, however, because it provides a
reference point and provides a great deal of insight into how best to use the material practically.
Well I now see what was going on when I tried to form silver azide on a stirred suspension of DDNP particles in water. I described what I observed
briefly here, at the bottom of the post, http://www.sciencemadness.org/talk/viewthread.php?tid=439&am... When the sodium azide was added there was gas produced. At the time I thought it
was hydrazoic acid, but I never got the symptoms of hydrazoic acid exposure/poisoning that I normally do when I do something foolish with azides
indoors. It turns out it was nitrogen gas which was being produced. From, "Primary Explosives", by Matyas, "It (DDNP) also reacts with sodium azide
forming the sodium salt of 2-azido-4,6-dinitrophenol and liberating nitrogen. This reaction may be used for analysis. The quantity of DDNP in a sample
is determined from the quantity of nitrogen produced."
[Edited on 26-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
DDNP Electrostatic Sensitivity
Here is another reference for DDNP electrostatic sensitivity. The attached table was taken from the Matyas text.
DDNP is significantly sensitive to electrostatic discharge, but it would seem that it wouldn’t normally pose the same hazard as most other primaries
would if they had similar static sensitivity. From Matyas, “Upon ignition, unconfined and unpressed DDNP burns like nitrocellulose even in
quantities of several grams. It does not ignite or detonate under water even when initiated by a blasting cap.” The text, “Military
Explosives”, also states that a spark falling into the open end of a detonator containing pressed DDNP causes only ignition and flashing of the
DDNP (no detonation).

[Edited on 27-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2.0g of Picric Acid Initiated with 0.20g of DDNP & 0.05g of Lead Azide (Unreinforced Configuration & 7.6mm X 6mm Bushing)
This test was the same as the last except that after the picric acid base charge was pressed into the 7.6mm id aluminum casing an aluminum bushing was
added (7.6mm X 6mm) to hold the DDNP and lead azide primaries. The bushing was made from a piece of 5/16" aluminum round stock. As in the last test
the top 0.5g of the 2g picric acid base charge was pressed using half the pressure of the bottom 1.5g (~20MPa versus ~40MPa). The DDNP and lead azide
were only hand pressed because a suitable loading dowel, which could be used with the press, for the 6mm id diameter bushing would have had to be
made. I really should have taken the time to make one, but the results were quite good in spite of lower primary density. Previous tests would
indicate that if the DDNP and lead azide primaries were pressed to higher density that there would be even higher output obtained from the picric acid
base charge.
The 2g picric acid base charge detonated with high output; similar to several of the earlier tests where a larger quantity of primary explosive was
used. I was really unsure of what the outcome of this test would be, and was happy to get such a good result.
Reducing the primary explosive charge diameter from 7.6mm to 6mm may not sound like a lot. However, if you consider that cross sectional area is
proportional to the diameter squared, the cross sectional area at the reduced 6mm diameter is only about 62% of what it is at the larger 7.6mm
diameter.
The hole on the right, in the witness plate shots, is from this test.
   
 
[Edited on 27-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
iso-DDNP
There is a less well known isomeric form of DDNP which is reportedly more temperature stable and possible to obtain from an easier synthesis. The
acetyl derivative of isopicramic acid which is the most convenient precursor may be obtained by direct nitration of paracetamol, acetaminophen, which
is a low temperature nitration under relatively mild conditions.
GB24409 describes a nitration of acetaminophen to the dinitro compound that is the acetyl derivative of isopicramic acid or more probably is
isopicramic acid.
The patent describes 15 parts acetaminophen being dissolved in 75 parts sulfuric acid at 0 C and nitration is accomplished by adding gradually with
cooling, 44 parts of a half and half mixture of sulfuric acid and 60% nitric acid.
After standing for several hours the nitration mixture is added to 200 parts of ice and the acetyl isopicramic acid precipitates and is filtered out.
Heating the acetyl derivative of isopicramic acid with dilute sulfuric acid should hydrolyse and split off the acetyl to produce the isopicramic acid.
See the Meldola and Stephens article page 1204, for the parallel. I am not certain that a deacetylation of the acetylisopicramic acid will occur the
same way, and the literature is unclear on this point. A later article opines that one acetyl is already split off from the "diacetyl" derivative of
acetaminophen when it is initially dissolved in the sulfuric acid. See page 1937 of the later Meldola article.
Diazotization carried out the same way as for DDNP, but using isopicramic acid provides the isomer of DDNP.
There has been some discussion before about possible nitrations of acetaminophen and what usefulness about that nitration may be, and this would seem
to be a relatively easy synthesis.
Relevant references are attached. I'm sure there are probably more pertinent references. It would seem like there should be some published study
comparing the properties of the two different isomers to identify their similarities and differences. What may be the difference in the DDT behavior
would be particularly interesting for example. Also what is the density difference and does there form a mixed crystal for the two isomers
cocrystallized.
The isomeric form of DDNP may be a better initiator, and its derivatives likewise could be better. So this is probably worth experimentation. I may
be rediscovering some lost art here, because I am not finding much of anything as references. But it looks to me like this proposed synthetic scheme
should work. An uncertainty involved is with the deacetylation of the acetylisopicramic acid, but I am hopeful that it will hydrolyze to isopicramic
on heating with dilute sulfuric acid. The literature is not clear on this point.
Attachment: GB24409 o-nitro-o-amido-p-acetamidophenol.pdf (166kB)
This file has been downloaded 843 times
Attachment: Pages from Journal_Chemical_Society_London pg1203.pdf (380kB)
This file has been downloaded 1020 times
Attachment: Pages B59-60 from PATR Vol. 2 B-C.pdf (225kB)
This file has been downloaded 1090 times
Attachment: Pages from Journal_of_the_Chemical_Society pg1935.pdf (423kB)
This file has been downloaded 744 times
An additional reference has been found, regarding what appears to be the deacetylation of the acetylisopicramic acid, Meldola and Hay article page
1482. But this is still being sorted out. Evidently the deacetylation is tricky and the isopicramic acid (or yet another isomeric analogue?) is
unstable. Comparison with isopicramic acid obtained by different method will be needed to determine if this is the same isopicramic acid or yet
another isomer.
Attachment: Pages from Journal_Chemical_Society_London pg1482.pdf (427kB)
This file has been downloaded 835 times
An additional reference describes a different approach for synthesis of isopicramic acid.
Attachment: Pages from The_Chemical_world pg327.pdf (247kB)
This file has been downloaded 823 times
The apparent nexus found in the literature are these last two articles by Meldola, evidently illustrating though not explicitly identifying two
similar and somewhat analogous syntheses of isopicramic acid. The Journal of Chemical Society article pg1482-1483 describes the isopicramic acid
gotten from nitration of acetaminophen, paracetamol to form the acetylisopicramic acid then hydrolyzed using sulfuric acid.
The article from Chemical World describes a slightly different approach involving a benzoyl derivative instead of the acetyl derivative of
aminophenol, however the nitration and hydolysis by use of sulfuric acid to leave isopicramic acid as the resulting end product seems to be analogous.
It would also seem likely the ammonium salt of isopicramic acid to facilitate isolation may be applicable for the acetyl as well as the benzoyl
derivative. The ammonium salt of isopicramic acid is likely stable and would probably be the desired form for isolation of the isopicramic acid. On
page 1482 the characterization of the isopicramic acid as having feebly basic properties is possibly a misprint or editing error, as my expectation
would be that the compound isopicramic acid is acidic, doh  , and would form the
salt that is ammonium isopicramate. Perhaps isopicramic acid is amphoteric? The description is confusing unless it is a typo. The isopicramic acid
likely is amphoteric similarly as is aminoacetic acid. , and would form the
salt that is ammonium isopicramate. Perhaps isopicramic acid is amphoteric? The description is confusing unless it is a typo. The isopicramic acid
likely is amphoteric similarly as is aminoacetic acid.
I am skeptical about the literature descriptions of structurally different 2,3 and 2,6 dinitro position identifications made in the early research
articles that are more cryptic in light of the statement in the Meldola article on the trinitro derivative on page 1937, identifying "the now well
known fact that p-acetaminophenol itself always gives rise to isopicramic acid on nitration". Well isopicramic acid is the 2,6-dinitro-4-aminophenol
compound,
http://books.google.com/books?id=rB0TAAAAYAAJ&pg=PA270&a...
so this statement doesn't reconcile with a lot of the previously published analyses already published if I am understanding the literature correctly.
Earlier Meldola misidentified (I think) the structure as 4,6-dinitro-3-aminophenol as being the ultimate product.
http://books.google.com/books?id=yJIFAQAAIAAJ&pg=PA157&a...
Obviously for "para" aminophenol (or acetaminophenol) the amino group would be opposite the OH of phenol at position 1 which would locate the amino at
ring position 4.
This has been a confusing review because it seems Meldola can't make up his mind about structure or is not being fully clear about what is updated
information changing what was identified differently and probably incorrectly IMO before.
The literature seems to show the several researchers were puzzled and not in complete coherent agreement. How well the jello got nailed to the tree
is not established. More specific experiments would be needed to further sort out and confirm what is actually occurring.
Picramic acid is the 4,6-dinitro-2-aminophenol compound, given the phenol OH at position 1 and the amino NH2 at position 2 on the C6H2 remaining ring
structure.
http://www.caslab.com/4_6-Dinitro-2-aminophenol.php5
Isopicramic acid simply has the 4-nitro and 2-amino groups of picramic acid transposed, the reverse order locations.
A few pages back Philou Zrealone was asking about iso-DDNP
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
[Edited on 10-6-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This looks very interesting. I am less than half way through the readings you provided, but I did do a search and this came up which looks to be the
same general topic:
http://www.sciencemadness.org/talk/viewthread.php?tid=2053
Properties could be very different from DDNP judging from what I learned about isomers in school. Sounds like it could be less sensitive, which could
be a good thing from a safety perspective or it may make it less suitable as a primary explosive since DDNP is already relatively insensitive as
primaries go.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yes it interesting because the iso-DDNP is reported to detonate violently at 190C ....not described to ignite and flash like guncotton or DDNP ....so
it may skip the DDT and just detonate, which would be good and would be an improvement over DDNP. The literature is confusing because it is pure
research and I think some of the analysis occurring is guessing about structure that is wrong. Obviously there would have to occur a rearrangement to
account for some of the structures misidentified as originating from a paracetamol which is a 1,4 position structure where the acetylated position is
the amino position 4.
The literature is describing reactions and structures that are puzzling to the reporters who are still sorting it all out, so it requires a kind of
oblique reading to follow and understand in corrected form what it is that is being described. An updated and corrected dissertation could probably
be produced from here at this forum. I don't know if an updated and corrected article has ever been done on this topic. I think the authors finally
figured out what their research findings were, but may have never published their final and corrected conclusions. And maybe nobody else has followed
up on this either, so it is left as unfinished work.
But with the relevance to iso-DDNP that seems like a huge oversight to leave the topic dead ended with incorrect and incomplete information.
Discovery of isopicramic acid is attributed to Dabney in 1888.
Of possible interest is that the common photographic developer metol can be nitrated to an N-methyl isopicramic acid. See US3641154 attached. It is
unknown what may be the result of a direct diazotization of the N-methyl isopicramic acid, or in the alternative if there is an easy demethylation to
provide isopicramic acid.
Attachment: Pages from Journal_of_the_Chemical_Society pg308.pdf (434kB)
This file has been downloaded 747 times
Attachment: US3641154 Methyl-isopicramic acid.pdf (209kB)
This file has been downloaded 757 times
[Edited on 10-6-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have been away for the last couple of weeks and where I am is not really an ideal location for experimenting. Do you think there is a possibility
that this isomer of DDNP could be a superior primary to DDNP itself? It seems like you think that it could be. I would find it very strange if that is
the case, and that it has been overlooked. The Chinese apparently use tonnes of DDNP in detonators every year. Seems unlikely that they would miss an
isomer with superior qualities. Still it would be interesting to do some experimenting and see what results we get.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It seems possible that the isomer could be a superior primary but that the economics of its use are not favorable in comparison to DDNP. It is also
possible that the explosive properties of the isomeric DDNP have never been investigated, or that the investigation was done and remains obscure
because of translation not done for the publication. I am not certain I recall correctly, but I may have read in PATR or another publication that the
Germans experimented some with the para isomer and preferred it to the better known ortho isomer.
Likewise for the other quinone versus phenol structure analogues described by von Herz in attachments to my reply to the question of PHILOU Zrealone
about the para isomer of DDNP a few pages earlier. The PATR article I think is in error about the instability of those compounds and may be a
misprint (perhaps "unstable" should read instead "usable"?), but I have no followup information. And the diagram posted by PHILOU Zrealone for the
para isomer structure is likewise also I think incorrect. The diagram shows a 3,5 dinitro isomer which is not para DDNP. So there is more to be
sorted out about these "errors" that I still think I am seeing in the literature and discussion even here. I sent a U2U to PHILOU Zrealone about this
thread updating. Here attached is the correct structure for the para DDNP isomer
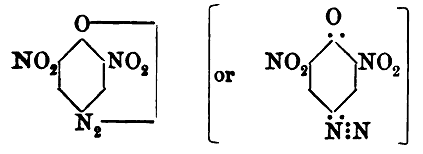
In fairness to Reverdin and Meldola I think most of their work is good and is correct, and it becomes clearer with the later articles of Meldola. I
think because there was simultaneous research and publication there was a too polite kind of academic courtesy operative for each avoiding repeating
verbatim the others work and as a consequence some of the material making most sense needed in regards to our topic of interest was not clearly stated
by either one in their published articles, which is our disadvantage. Meldola actually wrote a book that was specific about his extensive experiments
but I don't think there is an e-book.
There is nothing in the old literature to indicate that the explosive property reported for the para DDNP was something given due attention, and that
the para DDNP was not the object and end product desired but was being described only in passing as an intermediate in synthesis of other compounds.
The research focus seemed to be a priority of describing broadly the general chemistry for a class of compounds where the para DDNP was a lesser
interest as what was probably regarded a dangerous diazo compound to be avoided rather than investigated further so attention was given to moving
right along with mapping the reactions for the more mundane and safer compounds. Dye research seems the likely focus more than was their interest in
a new explosive. With the spotlight of more awareness later being given to DDNP the para isomer could have simply been relegated to academic
obscurity. So it is intriguing what is the real story not only for the para DDNP but also for the other patent compounds of von Herz.
So, Yes, von Herz was probably Austrian 
He probably appreciated structural correctness for quinone
is a sine qua non 
And I still have to wonder if the N-methyl isopicramic acid would demethylate under diazotization to form the para DDNP as the same resulting product
as when isopicramic acid is diazotized. Nothing I have found in the literature provides any clue. Since Metol is ubiquitous as a film developer then
that would seem to be a valid precursor and too good to overlook, even though paracetamol as a starting material could be workable too.
Regarding the amphoterism aspect of these diazo compounds that I mentioned earlier, yes I believe there does exist amphoterism or similar property at
least in a qualified sense, and the ready solubility even for DDNP in concentrated HCl versus insolubility in dilute HCl shows this amphoterism for
DDNP, along with I think the reactivity of DDNP with certain alcohols like isopropanol which therefore can't be used for recrystallization of DDNP for
reason of that reactivity.
Attachment: Isopicramic Acid Pages 243-4 from PATR Vol. 1 A.pdf (169kB)
This file has been downloaded 1050 times
Of related interest is that it is possible but difficult to introduce an additional nitro group in the case of an isomer of DDNP so that a 2,3,5
trinitro diazo variant is produced, but the compound is chemically reactive and probably unstable in comparison to the dinitro variants. See attached
article by Meldola and Hay as referenced near the end of the PATR excerpt. Also attached is the Meldola and Reverdin article where some revision
about structures is made if I understand correctly is a shared impression of Federoff conveyed in the PATR excerpt. It is understandable there could
be some variations of structure that would be confusing to the reader trying to track these various isomers that would seem possible and sort it out.
I know I suffer from "structure confusion" just reading about all these variants, like the fun never stops....but it gets there eventually.
Attachment: Meldola and Hay Pages JCS Vol95 part2 pg1378.pdf (478kB)
This file has been downloaded 1082 times
Attachment: Meldola and Reverdin pages JCS 103 part2 pg1484-94.pdf (766kB)
This file has been downloaded 1068 times
Yeah it gets complicated, which is another factor in favor of the para DDNP being not given attention. It was one of several variants that turned up
in a complex mapping of related compounds and not nearly as straightforward as the more well known DDNP.
The most interesting aspects of this old research being referenced is the para DDNP isomer and the dinitroquinone diazides patented by von Herz. Both
likely have value even though not having received much attention. It seems ironic in a way that there would not be more attention to such energetic
materials because they are "green" energetics and that has been so much a point of environmentally conscious motivation and "salesmanship" even though
IMO it is a disproportionate concern that is an irrelevant concern for probably most outdoor applications or occasional use scenarios as a practical
matter of concern as a source of environmental pollution significant enough to justify being mitigated.
The usual DDNP being used as an initiator was evidently patented first by von Herz according to the application date for the German patent DE373426 a
couple of days short of a full year before the Dehn patent filing in the US1404687. The Dehn patent issued earlier but the application date is the
invention publication, so von Herz gets credited.
And the associated von Herz patent GB207563 for the variants is what is more interesting still particularly with regards to the resorcinol related
compound potassium salt, but also interesting for others. The polyvalent phenols producing analogues of DDNP which retain an acidic hydroxyl and form
salts that are energetic makes possible nitrated phenol diazo compounds which are as powerful or more powerful initiators than lead azide.
There was earlier a post about the analogous reduction of styphnic acid
http://www.sciencemadness.org/talk/viewthread.php?tid=433&am...
and in the post following
http://www.sciencemadness.org/talk/viewthread.php?tid=433&am...
I attached the patent US4246052 and other patents.
For convenience I will attach again US4246052 here as this ties in nicely with the precedent earlier art von Herz patent GB207563 and supplies
additional information. This may provide an alternative route to the same nitrated resorcinol diazo derivative analogue or a variant of what is
earlier described by von Herz gotten by a different method in GB207563.
Attachment: DE373426 Original DDNP patent von Herz.pdf (179kB)
This file has been downloaded 761 times
Attachment: US4246052 SnCl2 reduction of Styphnic Acid and DDNP analogue therefrom.pdf (240kB)
This file has been downloaded 799 times
Attachment: GB207563 Diazidodinitrohydroquinone.pdf (371kB)
This file has been downloaded 705 times
Hopefully the nexus is obvious about these assorted references showing additional possible diazo compounds analogous to or variants of DDNP, some of
which are also capable of forming salts with metals and result in energetic compounds that are initiators more powerful than either DDNP or lead
azide.
There are potentially useful nitrated benzene diazo compounds obtainable from precursors for diazotization analogous to picramic acid, but resulting
in different and in some cases better initiators than DDNP. Some of the precursors may be similar in difficulty to prepare as picramic acid, and
others may be easier or more difficult. DDNP is simply one member of a general class of energetic materials where DDNP is one material most commonly
known, but other similar compounds are possible. DDNP illustrates a general idea as basis for variants that may be substantially better initiators
than DDNP itself. Von Herz identified several examples.
The lead salts reported to exist in both neutral and basic form also makes possible the formation of double or multiple salts and clathrates which
could be based upon such basic lead salts and would be more powerful than the multiple salts or clathrates derived from basic lead picrate.
[Edited on 12-6-2014 by Rosco Bodine]
|
|
|
Thanatops1s
Hazard to Self
 
Posts: 54
Registered: 24-6-2013
Member Is Offline
Mood: No Mood
|
|
In attempting a DDNP synthesis, everything seemed to proceed as it seems to right up until the diazotization. After adding the initial H2SO4 it turned
brownish(only took a small amount too). After adding NaNO2 solution I did notice a darker brown precipitate how after filtering, the precipitate was
basically absorbed and became inseparable from the filter paper other than a miniscule amount.
I have saved all filtrates and precipitates just in case anything is salvageable.
My guess is that I added too much acid right before the addition of the sodium nitrite solution but I'm not positive.
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
Sounds like low yields. Each step is nonideal and excessive losses leaves little at the end. Read the threads more with the intent on higher yields.
|
|
|
dave321
Harmless

Posts: 45
Registered: 22-11-2012
Member Is Offline
Mood: No Mood
|
|
spherical ddnp
this paper was posted by hennig in the
Short question / quick answer - Thread
I think it should be in this thread also if it is not already,
a very good paper.
I am a bit surprised they initially used sodium sulphide to produce sodium picrate and then more sodium sulphide for the reduction to sodium
picramate.........any comments?
Attachment: Synthesis and characterization of spherical 2-diazo-4,6-dinitrophenol (DDNP).pdf (1MB)
This file has been downloaded 1302 times
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
That article seems like alchemy, turning DDNP into lead azide as one would like to turn lead into gold. Though it promises great performance
improvements, it has a horrible grasp of the language. And the sodium picrate from sodium sulfide still leaves me scratching my head. Hello H2S.
Although I didn't follow that path (I used the standard NaOH) and I didn't have the specific crystals that were described for the sodium picramate, I
got poor results. The product seems to be degraded by the p-cresol. 8g of DDNP turned into 1.6g of needle crystals and some muddy stuff that isn't
free flowing nor burns with a whoomp, just a slow deflagration. The cap I loaded it in looked like a smoke b--b the way the smoke just jetted out
when ignited. I dissolved p-cresol in acetone along with the purified 1.6g DDNP and afterward got more of the muddy stuff and <.1g of needle DDNP.
I'm suspecting there is a reason why that article hasn't risen to the top before. Seeing how many in industry are hopeful of the green DDNP to
replace other heavy metal primaries, they would have already adopted it. Perhaps just a chinese supplier of cresol just selling snake oil. Maybe
Hennig Brand could give it a try if he hasn't already to see if my results are flawed somehow. He seems to be hopeful for DDNP like myself and tired
of making elaborate caps. Maybe this was just a first attempt with better results later. I'll probably give it another attempt, but not at the
moment.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have seen where sodium sulfide is specified to neutralize picric acid a couple of times before already. Check out the process block diagram below. I
have also attached the article it came from, sorry, so far I have not found the English translation of it. I guess it could be run through a
translator though. It seems like they are really intent on keeping only sodium sulfide and no hydroxide in the reaction mixture, which is interesting.
F-1 in the block flow diagram is their specially made crystallization modifier.
Remember most of this work is geared towards industrial processes. Methods used for industrial processes are often not nearly as suitable for small
scale.
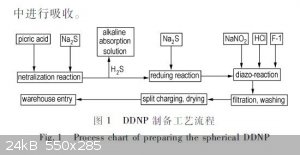
Attachment: Manufacturing Technology for Spherical DDNP (Chinese).pdf (1.7MB)
This file has been downloaded 940 times
With regards to the usefulness of DDNP in caps, I think there are still a few tricks that we don't know about yet which would likely make DDNP more
effective and easier to use as an initiator. It would be nice to see exactly how the Chinese make their DDNP detonators. Also, mass produced
reinforcing caps are much less expensive (time, energy, etc) per unit than reinforcing caps made one at a time at home. Is DDNP an effective initator
of secondary explosives? I think so. Can DDNP function as a direct replacement for lead azide? I would say definitely no.
[Edited on 19-8-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
For what its worth, this is why my son is learning mandarin. I'm afraid the technologists of the next age will be asian, whilst we aged curmudgeons
linger in the west like a bunch of bitter WASP's.
Here's the attempt I've made at translating that file. It gets complicated and the translator has a hard time, but it's readable-ish. The same
authors as the earlier article, different journal. I wonder if F1 is still p-cresol.
[Edited on 20-8-2014 by roXefeller]
Attachment: Preparation of spherical DDNP study.docx (166kB)
This file has been downloaded 704 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here is a pdf conversion for the word file
Attachment: Preparation of spherical DDNP study.pdf (170kB)
This file has been downloaded 1541 times
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Here is an article in English about spherical DDNP. The crystal modifier here is p-cresol (4-methylphenol), supposedly it can be synthesized from
toluene + sulfuric acid to create mainly p-sulfonc acid, which can be heated with KOH to produce the p-cresol. Not sure though if oleum is needed for
efficient sulfonation. :-)
If true, table 1 and 2 seem really promising, lol, 2800 initiations performed in total, poor student that took the project. :-)
Attachment: Synthesis and characterization of spherical 2-diazo-4,6-dinitrophenol (DDNP).pdf (1MB)
This file has been downloaded 819 times
[Edited on 20-8-2014 by nitro-genes]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
nitro-genes, that was just posted a few posts up. roXefeller and Rosco, thanks for the translation and pdf. Here are a couple more articles I dug up
for the DDNP collection.
Attachment: Structure and Bonding in DDNP.pdf (75kB)
This file has been downloaded 2026 times
Attachment: Theoretical studies on DDNP derivatives aimed at finding superior propellants.pdf (318kB)
This file has been downloaded 725 times
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
..
9
10
11
12
13
..
33 |