| Pages:
1
..
13
14
15
16
17
..
27 |
detonator
Harmless

Posts: 29
Registered: 12-4-2011
Location: Maoming City, Guangdong Province, China.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Formatik  |
An hour in the library sometimes saves hours and days of work in the lab, but it can also save extremities. Here was used far too large of an amount
of a material which they knew very little about. In Gmelin a citation for a nickel hydrazine perchlorate, [Ni(N2H4)2(ClO4)2], pale-blue precipitate
made from standing of aq. Ni(ClO4)2 with N2H4, describes it as extremely explosive and that even in aqueous dilute suspensions it explodes. There is
also a reference (H. Ellern, D.E. Olander, J. Chem. Educ. 32 [1955] 24) which claims that the nitrate (which has been investigated as a primary),
Ni(NO3)2.3N2H4, as a moist compound occasionally deflagrates spontaneously, but that the dry salt can explode spontaneously. |
Actually,the molecular formula of Nickel hydrazine perchlorate is [Ni (N2H4) 5] (ClO4) 2, In a paper published in JACS.The title is "Ionic Polymers
as a New Structural Motif for High-Energy-Density Materials",Received: October 13, 2011,Published: December 17, 2011。
The thesis describes the NHP molecules and the crystal structure obtained by X-ray diffraction.
[Edited on 26-4-2014 by detonator]
Attachment: NHP-JACS.pdf (780kB)
This file has been downloaded 1222 times
[Edited on 26-4-2014 by detonator]

|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Some hydroxylamine and hydrazine complex and hydrates
The advantages of hydroxylamine complex or hydrates in a compound give higher gas production, and better oxygen balance than amine/hydrazine
complexes. Hydroxylamine also give greater densities. Hydrazine's complexes offer good gas production, extensive hydrogen bonding, and increase the
heat of formation.
Complexes
https://docs.google.com/viewer?url=patentimages.storage.goog...
This patent show the interesting compound -
[Mg(NH2OH)4]2+ salt of perchlorate. The nitrate salt is also interesting. Li(NH2OH)2 [ClO4]- is
also synthesized with specific impulse estimated to exceed ammonium perchlorate.
The procedure is not much different of making the hydrazine complexes. In this patent - https://docs.google.com/viewer?url=patentimages.storage.goog...
This compound detailed the synthesis of [Al(N2H4)6]3+ [ClO4]-3. and also
[Mg(N2H4)2]2+ salt of perchlorate.
Hydrates
http://pubs.acs.org/doi/abs/10.1021/i360030a021
I need someone to help me un-lock this file since I only saw the 1st page. Maybe important data like densities are given.
Anyways, This paper gave some synthesis of some hydroxylamine hydrate of some hydroxylamine salts, such as the nitrate salt
[+NH3OH] [NO3]-•[NH2OH] and the monohydrate and dihydrate of hydroxylammonium perchlorate
[+NH3OH] [-ClO4]•[NH2OH] and [+NH3OH] [-ClO4]•[NH2OH]2 respectively
These salts are described in the abstract to be less hygroscopic.
Hydroxylammonium-Hydroxylamine dinitramide is detailed in this paper http://link.springer.com/article/10.1023%2FA%3A1011362427996...
The compound contain both neutral and zwitterionic hydroxylamine moieties involved in the hydrogen bonding scheme. This structure the hydroxylamine
exists in its three possible forms: protonated, neutral, and zwitterionic.
[NH3OH]2 [N(NO2)2]2•[N+3OH]•[NH2O-]
Klaptoke has synthesis few hydrazine hydrate of hydrazine compounds such as this one.
http://www.ncbi.nlm.nih.gov/pubmed/11421707.
5,5’-azotetrazolate dihydrazinate
(+N2H5)2 [(CN4-)2N2]•(N2H4)2 Containing nearly 86% Nitrogen
by weight.
he also made hydrazine azide hydrazinate https://www.google.ae/url?sa=t&rct=j&q=&esrc=s&a...
(+N2H5)(N3-)•(N2H4) or empirically N7H9, contains 91.5% nitrogen by
weight.
Klaptoke also synthesized moni and dihydroxylammonium 5-Nitriminotetrazolate.
http://onlinelibrary.wiley.com/doi/10.1002/zaac.201100479/ab...
All compounds in this paper has practically no use due to high sensitivity or low detonation velocity, except for 1 compound - dihydroxylammnoium
5-Nitriminotetrazolate hemihydrate.
[(+NH3OH)2 (CN4NNO2)-2]2•H2O
This compound have a detonation velocity higher than HMX while having lower sensitivity. This compound is also easy to make. 5-Amino-tetrazole
nitrated by 100% Nitric acid produce 5-nitriminotetrazole and upon addition of hydroxylamine solution gives this product.
EDIT(woelen): Fixed error in formula on request of DubaiAmateurRocketry
[Edited on 8-5-14 by woelen]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Molecular and Crystal Structure of a New High Energy Density Material: Aminoguanidinium Styphnate
www.google.com/url?url=http://www.mdpi.com/2073-4352/2/1/34/pdf&rct=j&frm=1&q=&esrc=s&sa=U&ei=eyijU8mPKJPLsAS8oIGIDw&ved=0CBkQFjABOCg&usg=AFQjCNG
O9gQHr4qfmmqokdeKsFaMzLxC-Q
.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
studie
Interesting post. The entire PDF file, I have translated and studied. I found information on the velocity of detonation or a detonation pressure.
Maybe I missed.
LL
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
An oddity
Attachment: Energetic cocrystals of DADP.pdf (2.6MB)
This file has been downloaded 1001 times
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
HANP
Interestingly attach test results HANP 1200mg / 300mg LA. (Hexamine nickel perchlorate) R8 = ETN 650mg / 300mg LA.

|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
TeACP and HMTA
I see that there are a lot of experienced chemists. Anders, PHILOU, Franklyn, Rosco, Dubai .. I have a question: TeACP
(tetraminecopper(II)perchlorate) and HMTA (hexamethylene tetramine) can create complex along together? The ammonium hydroxide solution (25% in aq.)
..? What about created? Created anything? And if so: What? Laboratory test Melting points to a new (unknown) compound.
Thanks, LL
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I think the semicarbazide complex of nickel perchlorate may be interesting and may not have the dangerous instability of the hydrazine complex.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NHN
I see that the substance NHN running through thread. NHN can detonate speed of 7000 m/s. But it has several conditions. Here are my experiences.
Laboratory of Liptakov do research. NHN no detonated 6 mm in diameter. Only 8 mm. Copper tube 8/10 mm. It should be progressive stamping. 1.7 g/cc 400
mg + 1.3 g/cc (400) + 1.0 g/cc + 0.6 g/cc. The following is a resistor. The following is 0.6 g/cc 400mg. The following is a seal. A total of 2 grams.
That is the minimum. Better to 2.4 grams total. Hydrazine is volatile and very toxic. Further attempts LL will not be repeated.

|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  |
I see that there are a lot of experienced chemists. Anders, PHILOU, Franklyn, Rosco, Dubai .. I have a question: TeACP
(tetraminecopper(II)perchlorate) and HMTA (hexamethylene tetramine) can create complex along together? The ammonium hydroxide solution (25% in aq.)
..? What about created? Created anything? And if so: What? Laboratory test Melting points to a new (unknown) compound.
Thanks, LL |
Hi LL,
It seems that HMTA (C6H12N4) does form complexes (Me(HMTA)2X2) with several metals like Me=Ni, Co, Mn (and maybe Zn); X being Cl or NO3.
Those complexes are sometimes linked to water ligands.
HMTA also form a complex with silver with one HMTA unit per Ag atom.
I haven't seen references of perchlorate anion but it is a good idea, even better would be nitroformiate anion...also as a special cyclonan variant
with NH4C(NO2)3 and (if it exists?) Cu(NH3)4(C(NO2)3)2 ;-)
It is plausible that HMTA will set some NH3 (gas) free from the Cu(NH3)4(ClO4)2.
For OB reasons it might be usefull to try higher valences of Ni and Co (Ni(3+) and Co(3+) except if they are too oxydant for the NH3 and HMTA ligands
(HMTA being a discrete form of methanal and amonia).
6 CH2=O + 4 NH3 <--==> C6H12N4 + 6H2O
So to try out!
Ni(HMTA)2(ClO4)3
Co(HMTA)2(ClO4)3
Ni(HMTA)2(C(NO2)3)3
Co(HMTA)2(C(NO2)3)3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Ni
Philou, you write too much (from my perspective) scientifically. I try to Ni(HMTA)2(ClO4)3
Thank you for the advice. Maybe arises LL-PHIL / supercyklonan...........
LL
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  | I see that the substance NHN running through thread. NHN can detonate speed of 7000 m/s. But it has several conditions. Here are my experiences.
Laboratory of Liptakov do research. NHN no detonated 6 mm in diameter. Only 8 mm. Copper tube 8/10 mm. It should be progressive stamping. 1.7 g/cc 400
mg + 1.3 g/cc (400) + 1.0 g/cc + 0.6 g/cc. The following is a resistor. The following is 0.6 g/cc 400mg. The following is a seal. A total of 2 grams.
That is the minimum. Better to 2.4 grams total. Hydrazine is volatile and very toxic. Further attempts LL will not be repeated.
|
Nice work LL!
It is a long time I play with Ni(N2H4)3(NO3)2.
I have tested also Co(N2H4)3(NO3)2 (stable pale Brown/amber powder with comparable properties to pink lilac NiHN) and Cu(N2H4)2(NO3)2 (turquoise blue
precipitate)...
This last one is highly unstable probably owing to oxydoredox potential of Cu(2+) vs hydrazine just like Ag(+) and takes fire spontaneously while
drying...most of the hydrazine being destroyed during process.
@LL and others,
I highly do not recommand the use of copper detonator in direct contact with NiHN (better use a plastic straw interface)...I suspect a Cu detonator
containing NiHN/SANC was involved in an unwished detonation of a N2H5NO3 pipe firework that occured at home. It is possible that NiHN exchanged
partially for Cu metal or that the mildly acidic N2H5NO3 reacted on its side of the detonator with the Cu oxyd-hydroxyd layer to make transient
Cu(N2H4)2(NO3)2, heating and detonation.
I have improved the performances of NiHN by admixture of silver acetylide nitrato complex (from neutral proces AgNO3/C2H2/water). Both dry precipitate
do mix nicely and are stable towards each other.
Ni(N2H4)3(NO3)2 + Ag-C#C-Ag.xHNO3.yAgNO3 : Mix by weight 2/1 to 3/1.
That way the flame/impact sensitivity of SANC and short D2D distance benefits from the high VOD and power of NiHN while SACN increases synergetically
the sensitivity and reliability to detonation of NiHN --> reduction of the critical mass of detonation.
Never try Ni(N2H4)3(ClO4)2 it is very sensitive explosive even in water solution a glass rod hit onto the surface of the container can set it off!
Use extreme caution with unknown explosive metal-oxoanion complexes of the kind and always start small and make heat and confinement testing (flame
test in open and in Aluminium foil wrapping).
NiHN burn like nitrocellulose in the open and minute amount but same amount in Al foil will detonate very strongly...
[Edited on 15-11-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Ni/Ag
Thank you for yourself and for others for pointing out NiHN + Cu tube. This is important. I read many times about 2 parts NiHN and 1 parts Mixture of
nitrate of silver acetylide complex. This is a good choice. Question for Philou: Diameter 6 or 8 mm? Weighed 0.6 or 1 or 1.5 grams? Thanks,
LL......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  | Thank you for yourself and for others for pointing out NiHN + Cu tube. This is important. I read many times about 2 parts NiHN and 1 parts Mixture of
nitrate of silver acetylide complex. This is a good choice. Question for Philou: Diameter 6 or 8 mm? Weighed 0.6 or 1 or 1.5 grams? Thanks,
LL...... |
@LL,
I must confess that I use almost always too much initiator and too big detonators  :
:
*5-8mm internal diameter
*2.5-4 cm long
*max density by vibration of clay-like consistency initiator powder and pressing by small powder increments via tooth-pick point
*so 1-3g
Why? Everybody has its own financial and practical limitations 
I prefer to waste 1 or 2g too much comparatively cheap initiator(*) (with 100% efficient detonation) than 100g (or more) valuable rare or labour
intensive HE main charge (TNB or N2H5NO3)...
(*)To me Ag, AgNO3 and CaC2 are very cheap; at that time was Ni, Ni(NO3)2 and HNO3 also cheap and easily accessible (I stil have a lot of Ni
nitrate).Nickel is less used in coin factories (allergies, environemental aspects, euro-legislation)...I remember not so long ago 50 belgian franc
coin was 7g of nearly pure Nickel (50BF = 1.25 Euros = approx 1.5$)...there is stil some Nickel Inside Euro-coins but not a lot  . .
I had also access to 80% hydrazine but 500ml was quite expensive (I stil have a few ml left...but I found a new provider and I own 5L 25% hydrazine
for a decent price and I have about 2kg of N2H5HSO4 in my personnal stock).
So I have never pursued the goal of optimum detonation quantity limit vs efficiency to spare money and allow me to make as much detonator from a given
quantity of initiator.
This would have been an interesting perspective and study...but it would have costed me time and money at a time I was looking for quick fun and
results...
--> yeah young people are usually impatient...
--> I do not escape to the rule 
--> I'm only human after all 
[Edited on 16-11-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NHN/Ag
Thank you for the perfect answer. Pity that ended Belgian coins. 8 mm cavity will be good. The content of 3 g too. Hydrazine is a problem. It is more
expensive and toxic. This is not the best way for amateurs. But the way it is. 3 g refill, that's pretty firing charge. I have the same opinion.
Rather large detonator (3), before spreading expensive charge. (100 and more). If the detonator safe, almost like NPED may need 4 grams in it. No fear
......
|
|
|
Monte Carlo
Harmless

Posts: 2
Registered: 25-11-2013
Location: here
Member Is Offline
Mood: Stochastic
|
|
Canadian nickels from 1981 or earlier are essentially pure nickel and are still in circulation.
http://en.wikipedia.org/wiki/Nickel_%28Canadian_coin%29
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Analogous to the inorganic coordination cyanide anion ,
K3[Fe(CN)6] , Potassium Ferricyanide ( Potassium Hexacyanoferrate III )
there is an inorganic coordination anion of Nitrogen Dioxide
K3[Co(NO2)6] , Potassium Cobaltinitrite ( Potassium Hexanitrocobaltate III )
The two could be co-precipitated from solution forming an oxygen balanced blend very close to a 2 : 3 molar ratio.
38 K3[Fe(CN)6] + 60 K3[Co(NO2)6] => 147 K2O + 60 CoO + 19 Fe2O3 + 228 CO2 + 294 N2
1.25 parts by weight of Ferricyanide to 2.71 parts by weight of Hexanitrocobaltate
In solution with an Ammonium salt it can undergo metathetical double replacement to become _
(NH4)3[Co(NO2)6] , Ammonium Hexanitrocobaltate III
mentioned in Explosive Properties of Metal Ammines here
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
Ammonium Hexanitrocobaltate III can be further reacted with hydrazine to replace Ammonia.
(NH4)3[Co(NO2)6] + 3 H2NNH2 => (H2NNH2•H)3[Co(NO2)6] + 3 NH3 ^
The result is a more energetic compound which also has excess oxygen.
4 (H2NNH2•H)3[Co(NO2)6] => 4 CoO + 30 H2O + 24 N2 + 7 O2
It occurs to me that formaldehyde will condense with the Ammonium and Hydrazonium cation of these complexes.
This would add carbon fuel to the oxygen rich compound.
One can also react Ammonium Hexanitrocobaltate III with an organic amine base to replace Ammonia.
(NH4)3[Co(NO2)6] + 3 CH3NH2 => (CH3NH2•H)3[Co(NO2)6] + 3 NH3 ^
This results in an energetic compound with a negative oxygen balance.
2 (CH3NH2•H)3[Co(NO2)6] => 2 CoO + 6 CO + 16 H2O + 9 N2 + 2 H2
Instead of Methylamine, using Dimethylamine yields a compound having poor oxygen balance but a very large molar gas volume.
2 [(CH3)2NH•H]3[Co(NO2)6] => 2 CoO + 12 CO + 10 H2O + 9 N2 + 14 H2
Here I thought I was on to something new and it turns out all this had been investigated back in 1909 !
Attachment: Studies on the Cobaltinitrites , Jnl of The Chem Soc (Transactions) 1909 - pg 1562.rtf (23kB)
This file has been downloaded 742 times
From here ( in .djvu , .pdf , and various other formats )
http://ia902608.us.archive.org/31/items/journalpt295chemuoft
I've written before that judicious blending of two compounds can remedy the shortcomings of either.
Combining a compound deficit of oxygen with one having a surplus yields an oxygen balanced composition.
7 (CH3NH2•H)3[Co(NO2)6] + 8 (H2NNH2•H)3[Co(NO2)6] => 15 CoO + 21 CO2 + 123 H2O + 79 1/2 N2
Nearly in the ratio of 5.4 parts by weight of the methylamine to 6.2 parts by weight of the hydrazine variant.
14 [(CH3)2NH•H]3[Co(NO2)6] + 52 (H2NNH2•H)3[Co(NO2)6] => 66 CoO + 84 CO2 + 558 H2O + 375 N2
Nearly in the ratio of 4.7 parts by weight of the dimethylamine to 6.1 parts by weight of the hydrazine variant.
These other coordination compounds are unstudied with regard to substitution with organic amine bases. Those in which the ammonia comprises the
complex anion itself cannot be so simply substituted. Formaldehyde will condense with a coordinated metal amine complex. Providing coordination is not
completely destroyed releasing the metal oxide, this will introduce carbon fuel in the compound.
Found in The Metal Ammines in the forum library here
http://library.sciencemadness.org/library/books/ATBOIC/atboi...
• Hexammino Cobaltic Nitrate , [Co(NH3)6](NO3)3 , pg 136
• Nitrato Pentammino Cobaltic Nitrate , [Co(NH3)5NO3](NO3)2 , pg 145
•1,6-DinitroTertaamminoCobaltic Nitrate , [Co(NH3)4(NO2)2]NO3 , pg 155
( it is also mentioned in Inorganic Synthesis vol 18 , pg 70 - 71 )
( both the cis & trans forms of [TetraammineDinitroCobalt III] Nitrate , [Co(NH3)4(NO2)2]NO3 )
• Hexammino Cobalt Nitrite , Co2(NH3)6(NO2)6 , pg 161
• Trinitro Triammino Cobalt , Co(NH3)3(NO2)3 , pg 162
• Ammonium Tetranitrito Diammino Cobaltate , NH4[Co(NH3)2(NO2)4] , pg 163
• Potassium Cobaltinitrite , K3[Co(NO2)6] , pg 164
Description
Fischer's salt (also called Fischer's yellow), Potassium Cobaltinitrite ( Potassium HexanitroCobaltate III ) a pigment known historically as Aureolin,
a.k.a. Cobalt yellow
http://www.jstor.org/discover/10.2307/1506479?sid=2110519426...
Sodium HexanitroCobaltate(III) reagent is used to test for Potassium and Ammonium ions, also forms the basis of quantitative determination of
Potassium.
http://cameo.mfa.org/wiki/Cobalt_yellow
http://en.wikipedia.org/wiki/Sodium_cobaltinitrite
http://en.wikipedia.org/wiki/Potassium_cobaltinitrite
Properties of Potassium Hexanitritocobaltate(III)
http://books.google.com/books?id=PpTi_JAx7PgC&lpg=PA759&...
http://books.google.com/books?id=IDsXBQAAQBAJ&lpg=PA473&...
Properties of Sodium Hexanitritocobaltate(III)
http://pubchem.ncbi.nlm.nih.gov/compound/159748#section=Chem...
http://www.lookchem.com/Potassium-hexanitrocobaltate-III-/
http://www.caslab.com/Sodium_cobaltinitrite_CAS_13600-98-1/
Preparation
http://chemistryfanatics.wordpress.com/2013/07/02/experiment...
http://www.studymode.com/essays/Preparation-Of-Sodium-Hexani...
Potassium Cobaltinitrite
Russian Journal of Inorganic Chemistry 04/2011; 56(4):501-505.
DOI: 10.1134/S0036023611040267
http://www.researchgate.net/publication/225112209_Potassium_...
Highest yield, smallest particle size, and highest purity of the product are attained by reacting Potassium Nitrite with Cobalt Nitrate in an acid
medium in the same way as in the synthesis of Sodium Cobaltinitrite:
2CH3COOH + Co(NO3)2 + 7KNO2 => K3[Co(NO2)6] + 2KNO3 + 2CH3COOK + NO^ + H2O.
All of the resulting Potassium Cobaltinitrite samples are hygroscopic.
The following reference found in Federov PATR-2700 , C-386
Cobalt Nitrite Complexes
When KNO2 is added in excess to a solution of Co(NO2)2 , acidified with AcOH, a yellow precipitate of potassium hexanitrocobaltiate ( potassium
cobaltinitrite ), K3[Co(NO2)6] forms. This compound is also known as Fischer's Salt or as a pigment Cobalt Yellow, formation of above complex can
serve as the basis of a volumetric determination of Co ion. When a solution potassium salt is added to sodium cobaltinitrite a yellow crystalline
precipitate potassium-sodium cobaltinitrite K2Na[Co(NO2)6]•H2 0 is formed. This complex, being practically insoluble in water, can be used as the
basis for detection of potassium ion.
References :
1)Gmelin, Syst Nr 58, Teil A (1932) , 400, 409 & 411
2)Kirk & Othmer 4 (1949) , 210-11
3)Gmelin, Syst Nr 58, Teil A, Ergänzungsband (1961), 513-15
4)CondChemDict (1961), 288
According to this , these complexes can serve as propellants.
Gas Generant Complex Oxidizers patent US 5962808
http://www.sciencemadness.org/talk/files.php?pid=91285&a...
Related posts
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Very nice Franklyn!
Just one remark on stability...
Nitrite/Nitro anion and ammonium or hydrazino ligands on a metal core might be very unstable.
NH4NO2 (ammonium nitrite) is already not stable.
NH2NH3NO2 (hydrazinium nitrite) turns into HN3 (hydrazoic acid)...this is observed when AgNO2 is mixed with N2H4...sensitive AgN3 results.
One may expect primary amine to be quite unstable too
R-NH2 + HNO2 --> R-OH + N2 + H2O
Secondary amines may transform into nitrosamine compounds
R2NH + HNO2 --> R2N-N=O + H2O
Tertiary amines may be stable
[Edited on 31-1-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
TeACP velocity
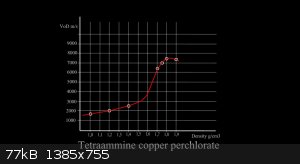
The my patent process is on a good way. Therefore, I can give important insights into the double salt TeACP. Measured by dent plate. Pure TeACP also
loses ammonia from the molecule. In the dry powder state very soon, during 3 days. In the pressed state at 1.8 g / cm3 as well. After 20 days (at
20C) begin to fall explosive properties. This can be prevented, in other words, importance to limit the loss of NHx.
With plasticizer PIB 2% + 2% oil 5W40. This is key. This mixture is safe for compression. This mixture has a zero oxygen balance. Detonation velocity
is very high. (graph) It is possible constructions SC or EFP devices. It is here in threads. Everything device (LL produce) is just filled with this
mixture. TeACP grain fraction is 0.05 -0.2 mm. Wet 5% H2O/NH3. Then mixed with a plasticizer, dry heptane, and pressed of 1.75 to 1.85 g / cm3.
Production TeACP is very easy, video here: https://www.youtube.com/watch?v=MDPa0Od3jws
Proven stability of 4.5 g pellet is 30 days / 20C. The power/VoD/ is the same as tetryl pellet. Detonation pressure at 1,7 - 1.8 g / cm3 is 20 - 25
GPa. (RDX 28 GPa). Initiation No.8.
For more details, I will discuss later. According questions.
...LL...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  | The my patent process is on a good way. Therefore, I can give important insights into the double salt TeACP. Measured by dent plate. Pure TeACP also
loses ammonia from the molecule. In the dry powder state very soon, during 3 days. In the pressed state at 1.8 g / cm3 as well. After 20 days (at
20C) begin to fall explosive properties. This can be prevented, in other words, importance to limit the loss of NHx.
With plasticizer PIB 2% + 2% oil 5W40. This is key. This mixture is safe for compression. This mixture has a zero oxygen balance. Detonation velocity
is very high. (graph) It is possible constructions SC or EFP devices. It is here in threads. Everything device (LL produce) is just filled with this
mixture. TeACP grain fraction is 0.05 -0.2 mm. Wet 5% H2O/NH3. Then mixed with a plasticizer, dry heptane, and pressed of 1.75 to 1.85 g / cm3.
Production TeACP is very easy, video here: https://www.youtube.com/watch?v=MDPa0Od3jws
Proven stability of 4.5 g pellet is 30 days / 20C. The power/VoD/ is the same as tetryl pellet. Detonation pressure at 1,7 - 1.8 g / cm3 is 20 - 25
GPa. (RDX 28 GPa). Initiation No.8.
For more details, I will discuss later. According questions.
...LL...
|
Is it normal that the VOD drops down between 1.8 and 1.9 g/ccm?
Dead pressing effect?
I will just point out that if TeACP is already very good, then by watching at the document proposed by Franklyn into Energetic Materials in
"tetraamine copper nitrate sensitivity" tread (posted on 30-1-2015 at 07:43)
==> The Combustion of the Salts of Tetramine Copper II
www.dtic.mil/cgi-bin/GetTRDoc?AD=AD0779649
In this document they expose the speed of combustion of various copper tetramine salts to be in the order BrO3(-) > ClO3(-) > ClO4(-).
So TeACBr > TeACC > TeACP but TeACP is much stabler.
Paradoxaly IO3(-) is the least interesting.
Sadly they haven't made the test for perbromate (BrO4(-)) and (meta)periodate (IO4(-)) or (ortho)periodate (IO6(5-))...
I suspect BrO4(-) (TeACPBr) might be even better than BrO3(-) (TeACBr).
For the periodates (TeACPI) they might be everywhere between BrO3(-) and IO3(-)...
TeACC = Cu(NH3)4(ClO3)2
TeACBr = Cu(NH3)4(BrO3)2
TeACI = Cu(NH3)4(IO3)2
TeACP = Cu(NH3)4(ClO4)2
TeACPBr = Cu(NH3)4(BrO4)2
TeACPI = Cu(NH3)4(IO4)2
[Edited on 3-2-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Brxx
Highest density (TeACP) was 1.91 g / cc. Technically. All shows on the effect of repressing. It is possible that a group of Br04, BrO3 and Br (IO3) is
better than ClO4. But research in this direction was not. I know the document two years. xx AD0779649.
... ...LL ...LL
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Nitrato Complexes of Manganese (III)
http://summit.sfu.ca/system/files/iritems1/4261/b1360529x.pd...
.
|
|
|
Keith Fletcher
Harmless

Posts: 29
Registered: 3-10-2014
Location: Eastern US
Member Is Offline
Mood: Brittle
|
|
I have Been looking into Nickel and Cobalt Hydrazine Nitrates. The reaction between ethanolic solution of Nickel or Cobalt nitrate with Hydrazine
Hydrate as well I am planning on making Titanium Nitrate (Ti(NO3)4) by reacting Titanium dioxide with Nitric acid. What I was wondering is if it is
possible to react this salt in solution with Hydrazine hydrate to form a compound that would share similar properties of NHN & CHN. The reason I
ask is that could not find any documentation on the reaction of Titanium Nitrate with hydrazine Hydrate. I am not shore of the mechanism that forms
metal Hydrazine Nitrates. I'm not even shore if Titanium Hydrazine Nitrate is a possible compound or if it would be stable. If Hydrazine cannot form a
complex with Titanium Nitrate then disregard this post.
Ti(NO3)4 + N2H4 = Ti(N2H4)(NO3)4
[Edited on 2-3-2015 by Keith Fletcher]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
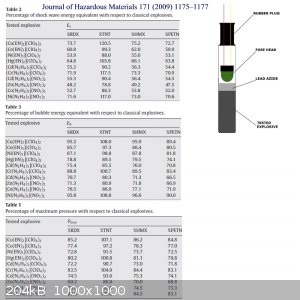
table
Here is graphically adjusted official studies, but titanium complex there is not. But the table can be useful... ...LL ...LL
|
|
|
Keith Fletcher
Harmless

Posts: 29
Registered: 3-10-2014
Location: Eastern US
Member Is Offline
Mood: Brittle
|
|
Interesting chart, Maybe it is possible. Do you think so. Titanium is a transition metal in the same period as Ni and Cu. Maybe Ti(III) can form an
Ammine complex that could be energetic.
My rule of life prescribed as an absolutely sacred rite smoking cigars and also the drinking of alcohol before, after and if need be during all meals
and in the intervals between them.
- Winston Churchill
|
|
|
| Pages:
1
..
13
14
15
16
17
..
27 |