| Pages:
1
2 |
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Some man just want to watch the world burn.
Do you realise what a terrible inefficiency that is? I file down every last part of the millimetre of the wall thickness and bottom thickness and do
all I can to add "inertial" confinement on the top.
Have you tried to initiate AN mixtures without Al/other sensitizer with that thing? I've seen quite and expensive charge fail, partially due to the
detonator being sealed with epoxy at the bottom.
|
|
|
Krakermanworks
Harmless

Posts: 9
Registered: 6-9-2013
Member Is Offline
Mood: No Mood
|
|
Thanks looks a bit 'hit and miss' though ill try nitrate/al/sulfur as that will give of more heat than nitrate/sugar.
|
|
|
Fuse
Harmless

Posts: 16
Registered: 25-2-2013
Member Is Offline
Mood: No Mood
|
|
@Ral123
The bottom is not sealed with epoxy.
The Heat-shrink tubing is heat up until she tighten to the capacitor.
I never tested AN without sensitizers.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1682
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
http://www.youtube.com/watch?v=eGW_lKX3OV4
ETN set off with fuse, supposing its 2mm visco 1cm/s standard (1.8mm??)
when this is possible, then overkill as in rocket candy cast into thin plastic tubes should be quite reliable as long as fuse to rocket candy is
ensured not to cause problems
not to mention i suggest that thinner layers of ETN packed in al foil is better compared to having one large blob of ETN, might be wrong..?
one thing tho: if using rocket candy you might want to add 0.5g ETN below the initiator, and not get the main charge above the initiator as this might
cause problems with the rocket candy burning at approx 1500*C, considering some secondaries are quite flammable (KClO3 / sugar for instance, if
anybody would ever attempt to initiate that?)
overkill is reliability
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Okay so to see how stable ETN is, I melted some, poured it on a flat block, and left it outside in the sun for a month (sheltered from rain). About a
quarter was uneffected, half turned yellow, and another quarter turned orange and crumbly. Some of the orange/ yellow stuff was actually really
sensitive, and detonated in little pops/ snaps when I touched it with a match. The whiteish stuff was pretty normal and didnt seem effected. The
changes are apparently from NOx related decomposition.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
What is the longest etn been stored for in different forms?
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Forms? I think its more about different levels of decomposition
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Forms as in cast, plasticized, pressed, compounded.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Daffodile  | | Okay so to see how stable ETN is, I melted some, poured it on a flat block, and left it outside in the sun for a month (sheltered from rain). About a
quarter was uneffected, half turned yellow, and another quarter turned orange and crumbly. Some of the orange/ yellow stuff was actually really
sensitive, and detonated in little pops/ snaps when I touched it with a match. The whiteish stuff was pretty normal and didnt seem effected. The
changes are apparently from NOx related decomposition. |
Couldnt the colors be juste related to how the ETN recrystallized?
I've been using hemolisis tubes as containers (intent: blasting rock) and the procedure was:
- Put ETN in
- Press ETN
- Put in boiling water.
- Repeat until desired load was attained.
I noticed at the beginning some recrystallized parts were clear, some were some kind of pink and maybe a darker color that I wouldnt go as far as to
call orange in the upper part of the tube.
Since I had to repeat the process to add more ETN and reach the desired load I noticed the colors and Crystal shapes changed quite a bit (as the cast
ETN was melted again).
That last part might be because after all the ETN was molten I tried once to leave it in the hot water until it was cold (which seemed to give large
crystals as one would expect).
Remove from the hot water and let it cool naturally and finally remove from the hot water and put it in the fridge.
In any case, I've left enough space to add some powdered ETN to ensure detonation. Holes were made through the caps and straws with one end closed
were hot glued in place to accomodate the detonators.
The idea of rapid cooling occured to me because it seems that for many explosives, smaller crystals are easier to initiate. Perhaps I am mistaken on
that point.
Anyway, now the hard part will be to find a rock and drill 15mm wide holes in it for my tests.
|
|
|
Brightthermite
Hazard to Others
  
Posts: 133
Registered: 26-6-2019
Member Is Offline
|
|
ETN Stability
Hate to bring this back up from the depths but I could not find a thread just on ETN storage stability and I wanted to report some findings.
I synthesized 14 grams of ETN using sulfuric acid ammonium nitrate method. 7 grams of this was well washed but not recrystallized. It was then placed
in an air tight pill bottle for 20 days. Opening that bottle (safely) thick orange brown gas was released, I assume this is from nitric acid left over
destroying the ETN. Only 2 ish grams of material was left. Interesting enough the material that was left was all completely white and did not appear
to have sublimed any. It performed well as if it was new. This being said, it seems un-crystallized ETN even if well washed is not able to be stored.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
And water is wet!
That is sort of a well understood fundamental, very un wise and dangerous to store ANY compound made via mixed acids to not be well washed and
recrystallized.
|
|
|
Brightthermite
Hazard to Others
  
Posts: 133
Registered: 26-6-2019
Member Is Offline
|
|
Follow Up
ETN made using AN/SA, over 1.5 years old, both from the same run. The one that is showing signs of decomposing was actually recryst twice using
acetone. I remember reading somewhere on here that if sodium bicarb is left in it can cause your ETN to decompose, I wonder if that is what's going on
here since the gas it not really a dark orange. Two recyst would have the ETN in contact with twice as much bicarb so..
And as XeonTheMGPony claims above, what actually makes decomposing explosives dangerous? In the case of ETN all I have seen it do is destroy the
product, make it under perform, and harder to set off. Are there some mixed acid made explosives that become more sensitive with acid left in the
structure?
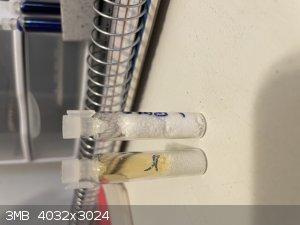 
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Brightthermite  | | what actually makes decomposing explosives dangerous? In the case of ETN all I have seen it do is destroy the product, make it under perform, and
harder to set off. Are there some mixed acid made explosives that become more sensitive with acid left in the structure? |
Yes, quite some. Self catalyzing decomposition processes leading to thermal runaways and kaboom.
You might want to research the history of the beginnings of modern explosives, the origins of scavengers for NOX such as urea, diphenhydramine or
centralizes being added to smokeless propellants, carbonates being added to dynamite "dope" and such.
That history includes the formation of several craters where explosives magazines, arsenals or manufacturing plants once stood. The odd USA battleship
blowing up. Austria banning all production of smokeless powder for a few decades. Subtle hints at what large quantities of acid tainted, decomposing
high explosives might do.
Download Tenney Davis' Chemistry of Powder and Explosives from the SM library and do some research.
http://library.sciencemadness.org/library/books/the_chemistr...
Phokin Naoum Nitroglycerin and Nitroglycerin Explosives also has basic information on neutralization, degradation and stabilizers.
http://library.sciencemadness.org/library/books/nitroglyceri...
[Edited on 11-4-2021 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
How clean was your Acetone? Any thing I use for my production is cleaned and distilled, I hate variables so I kill off as many as I can so I have a
known state to go back to if things go wrong, Acetone is very good at striping oils and such, so if you didn't buy lab grade (I sure don't) I can tell
you it had contaminants in it!
Next as Bert mentioned, very few things escape the notice of entropy, all ways use a scavenger. I use 3% by weight of urea for ETN.
|
|
|
katyushaslab
Hazard to Self
 
Posts: 81
Registered: 19-1-2021
Member Is Offline
Mood: precipitating
|
|
As Bert mentioned, decomposition can be self catalysing.
A few years ago I had a bunch of commercial "flash cotton" (nitrocellulose) stored damp in a cupboard, ignored for a few months. When I eventually
went to check on it, a good amount of it had turned into a kind of goop, there was a strong stench of NOx from it, etc.
I figure a small amount of it degraded, and started taking the rest with it - as more NOx is released, more acid can form, accellerating the breakdown
of the material. If this process generates sufficient heat... bad things happen.
|
|
|
Brightthermite
Hazard to Others
  
Posts: 133
Registered: 26-6-2019
Member Is Offline
|
|
Thats interesting, so the danger lies in the generation of heat. Not the acid some how making the explosives more sensitive.
My acetone is from the hardware store, so as clean as that is. Ill make a habit of distilling it from now on. Im also interested in using a scavenger
and doing some more 1 year plus testing.
Does anyone have any methods for telling if there ETN is pure and acid free. I have in my notes that the sample that is decomposing appeared to be
completely pure, it obviously is not. It frustrates me I was not able to tell. Maybe my recyst method is not great?
Dissolve in acetone, dump in water with about 5 percent bi carb.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I would suggest you do this instead for recrystallizing:
- Dissolve in acetone
- Add solid bicarb or possibly aqueous ammonia to the acetone and stir for at least 10 min.
- Crash in ca. 5% aqueous urea soln.
|
|
|
Fulmen
International Hazard
    
Posts: 1749
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
@Brightthermite: To be precise it's a runaway reaction driven by both heat and NOx. Short story:
Certain nitrate esters (like cellulose nitrate) are inherently unstable, very slowly decomposing to water, CO2 and NOx plus heat. The initial rate in
pure samples is completely negligible, however heat and NOx promotes further decomposition (evolving more heat and NOx). In small, properly stored
samples the heat and NOx will diffuse out too fast to do real harm. But in large amounts (and sealed containers like ammunition) the process can reach
runaway conditions. And even if it doesn't self ignite it can cause erratic performance in munitions.
I can't recall any cases where sensitivity was greatly affected, but you should always assume the worse.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Brightthermite
Hazard to Others
  
Posts: 133
Registered: 26-6-2019
Member Is Offline
|
|
@Fulmen thank you clearing up that it doesn't necessarily make the material more sensitive. I could see how a run away producing heat in a sealed
container with fully crystalized explosives could be dangerous.
@Microtek I believe ill try the ammonia, the problem with bicarb in acetone is the solubility is very low. So I think this makes it hard to get the
bicarb in contact with the acid. Is there a specific pH you go for when using ammonia? Is the solution of urea how you introduce the scavenger like
mentioned above?
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Take advantage of the water left after filtering. Dont dry unless you really want to know your yield at that stage. You are getting it wet again soon
enough.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
You should only aim for pH 7-7.5 otherwise you risk hydrolysis or other reactions (if you go to higher pH). The intention with the urea is to include
just a very small amount in the crystals to scavenge small amounts of NOx (in order to break the autocatalytic feed back loop).
|
|
|
Brightthermite
Hazard to Others
  
Posts: 133
Registered: 26-6-2019
Member Is Offline
|
|
@Herr Haber are you suggesting not to dry in hopes that water will help dissolve the bicarb?
I hope all this helps me get consistently more stable products. I have some samples that are 2 plus years old and showing no signs of decomposition
and others that are a half a year and smell of acid. Very frustrating.
Also pure ETN dissolved in any solvent should have a pH of around 7 correct? looking for more way to test purity besides smell.
|
|
|
| Pages:
1
2 |