krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
Synthesizing t-Boc protected derivative of PVA, many questions
I've been toying with different potential recipes for DIY photoresist. My current favored design is a chemically amplified resist based on PVA
(poly(vinyl alcohol)). For those who are unaware, a CAR works like this: a polymer is functionalized with a protecting group, and is mixed with a
photoacid generator (PAG), which as the name suggests, generates acid upon exposure to light. The acid then cleaves the protecting group (in this
case, t-Boc), rendering the polymer soluble in the developer. Basically, my question is how to functionalize PVA with the t-Boc group.
References are scant, but I did find one, which is unfortunately extremely vague. It says to react fully hydrolyzed PVA with di-tert-butyl dicarbonate
(boc anhydride) and sodium hydride in N-methylpyrrolidone. However, the paper itself says that this reaction has poor yields, and the authors are
(were?) looking for better options.
Not being satisfied, I decided to lookup the reaction used for functionalizing poly(hydroxystyrene), which is the polymer used in the original CAR,
APEX. Unfortunately, I wasn't really able to find anything on this either, save for even more vague references.
Anyhow, I have looked up information on how amino acids are protected with t-Boc, but the problem here is that I'm not sure if that would even work on
PVA.
Does anyone have any idea how I would I go about doing this? Even more preferable would be a method not involving dangerous/explosive and for that
matter, expensive substances such as Sodium hydride, or dangerous solvents (although NMP is not really that bad).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
This is a science forum, so please behave accordingly and cite the references you use.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
I'm sorry. . .I can give the link to the paper, but don't you think that's kind of abrasive?
http://proceedings.spiedigitallibrary.org/proceeding.aspx?ar...
|
|
|
hydride_shift
Harmless

Posts: 23
Registered: 25-7-2013
Member Is Offline
Mood: sp3 hybridised
|
|
The mechanism of Boc protection involves the lone amine pair attacking the carbonyl carbon to form a peptide bond, to protect the N terminus of an
amino acid. I don't know how your desired ester could form, and it would be far from trivial to do so.
You should look into benzyl esters, commonly used in C' terminus amino acid protection. These are cleaved by conventional strong mineral acids, and
more within the scope of a home lab.
refs ; jones j "amino acid and peptide synthesis" 2e oxford chemistry primers (undergrad peptide synth textbook)
[Edited on 15-1-2014 by hydride_shift]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2685
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Krf:
I think that the problem is that if people don't understand what you are asking about, IN DETAIL, it is nearly impossible to give a valid answer.
Making it worse is that you are supplying a number of terms together that don't make much sense (to me at least) in your question.
You talk about a DIY photoresist, but there are many types, and without some spepcifc reference to the type you describe, it is hard to answer.
Also, t-Boc groups are commonly used to protect amines, not acids, so that makes no sense to me. If you wanted to protect an alcohol polymer, you
might use tert-butyl esters, but not a t-Boc group. And I am not aware of them being light sensitive, whereas many other protecting groups are light
sensitive. Thus, it is hard to understand the question. And benzyl esters won't work for photo resists either.
And PVA and polyhydroxystyrene are entirely different types of polymers, so little will work the same between them. If you can give a scheme for
your chemistry or a link to something describing what you hope to be like, then it would be easier to help you.
|
|
|
krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
Dr. Bob, thank you for the feedback!
Quote: Originally posted by Dr.Bob  | Krf:
I think that the problem is that if people don't understand what you are asking about, IN DETAIL, it is nearly impossible to give a valid answer.
Making it worse is that you are supplying a number of terms together that don't make much sense (to me at least) in your question.
|
I understand. I will try to be more clear in the future.
Quote: Originally posted by Dr.Bob  |
You talk about a DIY photoresist, but there are many types, and without some spepcifc reference to the type you describe, it is hard to answer.
Also, t-Boc groups are commonly used to protect amines, not acids, so that makes no sense to me. If you wanted to protect an alcohol polymer, you
might use tert-butyl esters, but not a t-Boc group. And I am not aware of them being light sensitive, whereas many other protecting groups are light
sensitive. Thus, it is hard to understand the question. And benzyl esters won't work for photo resists either.
|
I understand that there are many different types of photoresists. I also know that t-Boc is generally used to amines, not alcohols. However, by virtue
of the fact that it has been used to protect alcohols, I know that it is possible to do so. Also, sorry if I was unclear, I did not mean to suggest
that I thought that t-Boc is light sensitive. It is, however, acid-sensitive, which is the point of the photoacid generator: it generates acid upon
exposure to light, which in turn cleaves the t-Boc group.
Quote: Originally posted by Dr.Bob  |
And PVA and polyhydroxystyrene are entirely different types of polymers, so little will work the same between them. If you can give a scheme for
your chemistry or a link to something describing what you hope to be like, then it would be easier to help you. |
I know they are different, but it is my understanding that the t-Boc group was substituted for the hydroxy group in the original APEX resist, so I
referred to that only because I also wanted to substitute the hydroxy group in PVA with t-Boc (sorry if that doesn't make any sense).
Thanks again
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
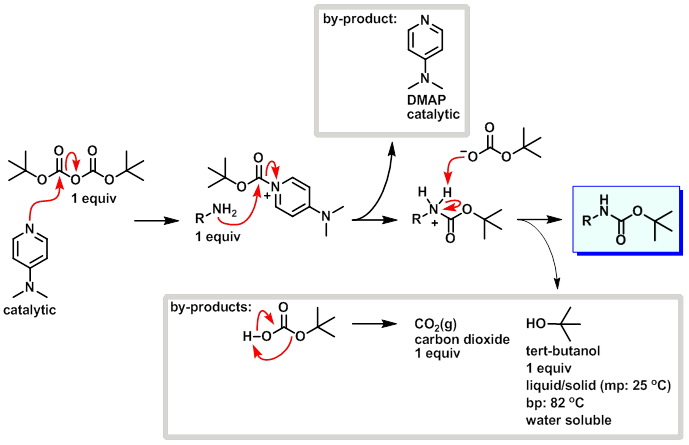
tert-Butyl carbonates can be made from alcohols by their reaction with Boc2O in the presence of DMAP (for example, see DOI:
10.1021/jo000257f and the references cited therein). See also the corresponding chapter in Greene's Protective Groups in Organic Synthesis
(Peter G. M. Wuts, Theodora W. Greene), though it is only a two page review. The Organic chemistry portal also gives a short literature review.
The reaction is not clean and predictable. In the case of a polyol, such as polyvinylalcohol, you will likely get a lot of cross-linking which might
make the polymer unmanageable and insoluble. Depending on the condition, you might get mostly internal carbonates instead of the tert-butyl
carbonates (polyvinylalcohol is essentially a polymeric 1,3-diol and can thus form six-membered cyclic carbonate units). I suggest you check the
literature for this specific case - likely it has been already reported.
Not at all, I do not think it is abrasive to cite references. How did you come to such a conclusion? Nobody will think you are rude, if you provide
proper references (it is a science forum after all!). On the contrary, if you don't cite references, members here will tend to believe you don't
really care about any good discussion and will lose interest in the topic. Also, because unless you supply references, nobody will know what you talk
about, as there is nowhere to read about the topic. Read the forum guidelines for more information.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Nicodem, he meant that your reply was abrasive. [delete this reply after reading]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Jeez, bfesser. Nicodem's just being facetious. I guess it takes a special kind of people to appreciate the humor.  [also delete after reading] [also delete after reading]
|
|
|
krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
MgO-ZrO2 catalyst for t-Boc protection of Alcohols. . .few question about application
I've posted before about my efforts to find a safe and effective means of synthesizing poly(vinyl t-Boc) from PVOH. Resources are rather scant.
However, I did come across an interesting paper today which, though it does not specifically refer to the reaction I'm looking for, does seem
promising:
http://onlinelibrary.wiley.com/doi/10.1002/aoc.2846/full?xml...
A quote from the article, ". . .we present herein an ecofriendly, chemoselective and simple O-tert-butoxycarbonylation of phenols and alcohols over
MgO–ZrO2 nanoparticles (NPs) under solvent-free conditions."
The authors mention multiple times that the process is highly selective to alcohols and has extremely high yields (up to 95% in some cases).
Obviously, the only way to find out is to try it, but, is there any reason why this wouldn't work with polyvinyl alcohol? Clearly, if there's
something I'm overlooking, there's not much point in trying the reaction if it is definitely going to fail. I can't really see why it wouldn't work,
but then again, if things were that easy I feel like there would be references elsewhere, and the very few references I have seen to making
esters of PVA all detail very complicated procedures. Is there a reason why this sort of method in the paper would not work with a polymer?
Unfortunately, there isn't really such a thing as "vinyl alcohol" so I can't try it on the monomer.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
One possibly problem here may be that the authors were applying a heterogeneous catalyst to a reaction with low molecular mass substrates. High
molecular mass polymers like PvOH or even the product poly(vinyl t-Boc), may adsorb very stongly onto the heterogeneous catalyst's surface and so
effectively poison it and render it ineffective.
For a heterogeneous catalyst to perform effectively, both the adsorption and desorption rates (first and last steps in a multistep chemical kinetics)
need to proceed at a decent pace. In this case, I would suspect the desorption would be very slow because the polymer's tendency to 'stick' to the
surface of the catalyst would be very strong.
You would be better off finding a way to prepare a vinyl t-Boc monomer first and then polymerise that, if that's possible. Alternatively, try to find
a homogeneous catalyst for you Pv-OH protection step.
[Edited on 12-4-2014 by deltaH]
|
|
|
krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
Thanks for the swift and helpful reply! That really clears up a lot. I have a few papers somewhere on single-component catalysts for Boc-protection,
so I'll look those up. Also, I have a paper somewhere around here which basically describes melting the Boc anhydride and putting whatever you want to
protect in it to protect it. Do you think something like that would work?
Second point, with respect to synthesizing the monomer. There is actually a paper describing this. Unfortunately it seems to require some pretty scary
and also expensive stuff as well as a bunch of equipment I don't have (glovebox for inert atmosphere for the polymerization). http://link.springer.com/article/10.1007%2FBF00955876
| Quote: |
A mixture of 12 g of t-butyl alcohol (0.16 mole) and 8.6 g of pyridine (0.11 mole) which was dried and distilled over KOH was added dropwise with
stirring into 10.4 g (0.1 mole) of vinyl chloroformate at 0 C. After half of the mixture had been added, the rest of the material is added as rapidly
as possible, keeping the temperature below 10 C. Ten minutes later, the flask was brought to room temperature, after addition of dichloromethane and
water the mixture was shaken then left standing overnight. The two layers were separated and the water layer was discarded away. The organic layer was
then washed twice with 15% HCl then twice with 20% HCl. The organic phase was again separated then dried over MgSO4. The distilled product is a
colorless liquid (77% yield).
|
Pyridine is not awful, but it's also less than ideal and not the easiest thing to come by. KOH is fine. Vinyl chloroformate is scary, expensive, and
very difficult to find. Chlorohydrocarbons are generally not my favorite things, though dichloromethane is on the less bad end of that. The rest isn't
that bad. Unfortunately, as mentioned, the polymerization seems to be difficult to perform.
| Quote: |
All polymerizations were carried out as described in Table 1 under inert atmosphere. The temperatures indicated in Table 1 were the temperatures
measured within the oil bath rather than within the polymerizing mixture. After polymerization, the polymer was dissolved in benzene and freeeze-dried
prior to precipitation. Alternately the polymer dissolved in toluene was precipitated in hexane.
|
Oh lovely, toluene and benzene, my favorite. So yeah, basically, any ideas on how I might go about that with less expensive reagents, and with more
amateur friendly equipment?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I will defer this to the organic chemists to discuss further who probably have better experience with using and protecting with the BOC group. I'm
more a catalysis kinda guy who dabbles in organic chemistry from time to time. You would do much better with the likes of nicodem on
this topic furthermore.
But this topic has given me an interesting idea, that is fusing urea and PvOH to possibly derivatise the polymer with carbonate. Be warned though,
urea decomposition releases copious amounts of ammonia!
[Edited on 12-4-2014 by deltaH]
|
|
|
Nicodem
|
Threads Merged
12-4-2014 at 07:56 |
krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
Seeing that the thread has been merged with the original one I made, I guess it would have been better to just bump that one than start a new one. I
apologize, but I know better for the future now!
After doing a little additional research, and reading back through the original replies, I think that Nicodem is right. . .
Quote: Originally posted by Nicodem  |
The reaction is not clean and predictable. In the case of a polyol, such as polyvinylalcohol, you will likely get a lot of cross-linking which might
make the polymer unmanageable and insoluble. |
As was said earlier, obviously polyhydroxy styrene is a completely different polymer than PVOH. However, a quick review of the literature and patents
seems to suggest that for positive resists (which is what I want), the t-Boc derivative of the monomer is first synthesized, and then polymerized to
ensure total substitution. There are also references to the same problem of cross-linking. In light of this, I think that synthesizing the monomer
first is the only viable method to do this. However, this does present a major problem it would seem. Namely that I have only found one paper
describing how to do this (the one already mentioned) and as I said, it requires pretty nasty stuff.
So, first question is: does anyone have any idea of how to make vinyl t-Boc in a more amateur-friendly way? Most preferable, starting with vinyl
acetate monomer.
Barring a good solution to that problem, I had a few other ideas. Unfortunately, vinyl alcohol monomer doesn't exist, otherwise that would be the
ideal way to go about this. However, there are a few options. T-boc is used primarily for protecting amines, right? Does vinyl amine exist? If so,
that seems like it would be an ideal precursor, since the protection of amines seems to be the most well characterized. The other option is to forgo
t-boc as the protecting group altogether. Apparently, vinyl trimethylsilane exists: http://www.sigmaaldrich.com/catalog/product/aldrich/661724?l... and can be bought (albeit at too high a price for me). This could be polymerized,
so I'd have poly(vinyl TMS) instead of poly(vinyl t-boc). Unfortunately, I have no clue about whether the polymerization of vinyl TMS has any
advantages over that of vinyl t-boc. Not to mention, my entire purpose here is in making a safe and non-toxic photoresist, and perhaps TMS might not
be so safe. Anyway, just a few ideas I had.
EDIT:
Well, I did a little bit of research, and found some promising data on the poly(vinyl TMS) (PVTMS) route. US Patent 3729454 describes the polymerization of vinyltriorganosilanes. On the subject of safety, firstly:
| Quote: |
Polyvinyltrimethylsilanes feature good compatibility
with tissues of the'living organism and are applicable in
endoprosthetics (cf. U.S. patent application No. 51,745).
|
Well that's promising!
The process described in the patent is somewhat confusing, but it seems to basically involve initiating the reaction with a lithium containing
oligomer of vinyltrimethylsilane. I'll have to re-read it to understand exactly what is meant. Anyway, this is exciting!
[Edited on 12-4-2014 by krfkeith]
|
|
|
|