| Pages:
1
2 |
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
reaction between thiocyanate and nitric acid
By accident I found a rather surprising reaction between thiocyanate ion and nitric acid. The experiment is very simple:
Prepare a very concentrated solution of NH4SCN or KSCN in water (e.g. 1 ml). Add 2 times its volume (e.g. 2 ml) of concentrated nitric acid (65% HNO3
by weight). Swirl the test tube.
When this is done, then a very pale pink liquid is obtained. This pale pink color may be due to some reaction product, but it might also be due to a
tiny amount of impurity in the reagents.
Next, carefully heat the test tube, while swirling. At a certain point in time, the liquid turns turbid and yellow and some gas is produced. Keep on
heating, until the production of colorless gas goes smoothly and a lot of a yellow solid is obtained. Then stop heating. The reaction continues. The
colorless gas most likely contains quite some NO, because in contact with air, brown gas is formed (which must be NO2). The liquid starts foaming.
After the reaction, there is a lot of yellow solid material. I added a lot of water to the test tube, so that all of the solid material is under water
and then allowed the material to settle at the bottom. The result is in the following picture:

I am wondering what this yellow solid is. I could not find info about this on internet. There are documents about oxidation of thiocyanate, but these
talk about formation of (SCN)2, which is colorless or further oxidation to sulfate and cyanide.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
My first guess would be sulphur.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Of course, I also checked that, but that is not the case. At first, the color is quite different, sulphur is much lighter and more greenish yellow.
This yellow color is more like ochre. I tried the experiment multiple times and in some runs it almost is orange, while in others it is like the color
shown in the picture.
Another thing is that the material is soluble in warm bleach. It slowly dissolves, giving a colorless solution. Sulphur does not dissolve in warm
bleach.
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
I thought how cyanates give some polymerization products on hydrolysis, analogous to that you could think that your yellow powder is:
EDIT: Thiocyanuric acid

I wonder if you can make thio analog of trichloroisocyanuric acid by acidifying your bleach solution of your yellow powder.
[Edited on 2-12-2013 by Satan]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
It seems that it can generate hydrogen cyanide, hydrogen suphide, etc.
http://incipiat.wordpress.com/2011/11/05/add-potassium-thioc...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
woelen:
Strange how such a simple experiment doesn't seem to be reported on the Tinkerwebs? Maybe there really are things the Government doesn't want us to
know about... 
Seriously, this is all a little baffling. Some oxidation reaction would be a safe bet with all that nitric present but which one? I presume the
evolved gas was not NO (== > NO2)?
The pink might be due to very small amounts of Fe3+ but I don't recall very dilute FeSCN(2+) to be pink: more light orange...
Intriguing, to say the least...
According to wiki:
"Thiocyanic acid is a chemical compound with the formula HSCN that exists as a mixture with the isomeric compound isothiocyanic acid (HNCS).[3] It
is the sulfur analog of cyanic acid (HOCN)."
HSCN must at least briefly exist in such an acidic solution as it is a medium-weak acid itself.
Maybe look for reactions of HNCS?
[Edited on 2-12-2013 by blogfast25]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Does this happen with all thiocyanates or just ammonium thiocyanate?
When nitric acid gives NO usually it oxidizes something such as in case of sugars and nitric acid.
Thiocyanate is pseudohalogen and can be oxidized to (SCN)2 but what you formed is probably also thiocyanic acid which is unstable. Here is one
document I found but I can't access it:
http://pubs.rsc.org/en/content/articlelanding/1897/ct/ct8977...
Edit:
Compound is perthiocyanic acid.
http://sulphur.atomistry.com/perthiocyanic_acid.html
Citing from google:
which he assumed was thiocyanic acid, and that this rapidly decomposed into hydrogen cyanide and a yellow, ...
Yellow what? Probably your compound.
Chemistry of Elements by A. Earnshaw and Norman Greenwood says that it is a polymerization product of isothiocyanic acid which is insoluble yellow
solid at room temp because it is unstable page 324
Formula is H2C2N2S3 according to them.
[Edited on 2-12-2013 by Random]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
You could try extracting it into ether, ethyl acetate, toluene, carbon disulphide, etc.
I doubt that it is sulphur, more likely a HNCS polymer, degradation product.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Assuming it is perthiocyanic acid it might be a nice way to get carbon disulphide by thermal decomposition of it but there might be also more toxic
byproducts.
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
Thiocyanuric acid? Check my previous post with image attached. I hoped that somebody would search for cas which is visible in this image.
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have looked up the compounds mentioned, both the trithiocyanuric acid and the perthiocyanic acid. It could be both of them, both being yellow/ochre
substances. I'll try to find more information myself.
The page, linked by ScienceSquirrel about formation of HCN and H2S most likely is under dilute conditions. The mix of KSCN and dilute HNO3 indeed is a
sensitive test for ferric ions, but this mix can also give HCN (I don't believe it can give H2S under such oxidizing conditions). In the concentrated
mix I had the conditions are very different.
I also tried the experiment with KSCN instead of NH4SCN. And yes, the yellow compound is formed with these as well. Btw. I had a little runaway, as
described below 
I took appr. 1 gram of solid KSCN and added so much water that it just dissolves. The solution was nearly saturated. To this solution I added appr. 3
times its volume of nitric acid (65% by weight). Immediately a fine white compact crystalline precipitate formed. I expected this, it almost certainly
is KNO3. So much precipitate was formed that the liquid changed into a slurry.
A few tens of seconds later, the liquid become pale pink and one minute after that, it was pale red/brown and remained like that.
Next, I carefully heated the liquid. The white precipitate quickly dissolves on heating of the liquid, the color intensified, it become nice
red/brown. At a certain point in time, the liquid turned turbid again and fine bubbles of gas were produced. At this point, I stopped heating. Slowly,
the reaction intensified and the liquid started foaming. It looked as if the foam would slowly run out of the test tube, but suddenly the reaction
accelerated much more and the contents of the test tube was sprayed against the wall and a cloud of brown gas was produced. A lot of the yellow/ochre
solid was produced, immersed in remains of the red/brown liquid, with faint brown fumes still being produced while sticking to the wall. It looked
like vomit, giving of brown fumes  and I had a lot of work to cleanup the mess.
But the result of this experiment is definitely confirmative. KSCN also produces the yellow solid. Next time, I will prepare a somewhat less
concentrated solution of KSCN. and I had a lot of work to cleanup the mess.
But the result of this experiment is definitely confirmative. KSCN also produces the yellow solid. Next time, I will prepare a somewhat less
concentrated solution of KSCN.
My next step will be preparation of a somewhat larger amount of the yellow solid, filter it and rinse with water and dry and see what properties it
has (e.g. heating it, mixing it with an oxidizer like KClO3 and see whether it makes a pyro-mix, dissolving it in amine, according to one of the
papers linked above). That is the next step for one of the evenings to come.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Take into consideration that thiocyanic acid is white crystalline solid which is unstable at room temp as it is mentoined in the book I wrote.
Pale pink color is interesting because I haven't seen it mentoined in the literature. Probably some intermediate in the decomposition.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
For the production of CS2, it seems a bit wasteful of thiocyanate. I guess you'd rather buy CS2 right away if you'd need it.
As a find it's highly interesting. Trying extraction into ether seems like a logical next step. I wonder what comes out. I'm too scared of
unintetional production of HCN to try the experiment myself at this stage.
I'm amazed of the large number of new finds you have posted recently, woelen. What were you trying to synthesize when you made this discovery?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
From the same site Random posted from, I found this entry for perthiocyanogen:
| Quote: | Perthiocyanogen, HC3N3S3, is a yellow, amorphous powder, insoluble in water, alcohol, ether and concentrated sulphuric acid; it can be obtained by the
action of chlorine or boiling dilute nitric acid on an aqueous solution of potassium thiocyanate. It is decomposed by heat according to the equation:
3HC3N3S3 = 3CS2 + 3S + H3C6N9 (Mellone.)
With concentrated potassium hydroxide solution thiocyanic acid is obtained. |
http://sulphur.atomistry.com/perthiocyanogen.html
My money is on this compound 
Credit to Random for finding this site... this compound was just below the link for perthiocyanic acid that he talks about above.
I tried to do some digging about what is 'perthiocyanogen', googling just turns up lots of dictionary entries?! Google patents turns up this:
"Cells having cathodes with thiocyanogen-containing cathode-active materials", US 4192912
There are references here and there in google scholar that mentions this stuff, but it doesn't seem well described/studied at all.
[Edited on 5-12-2013 by deltaH]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
I suspect that is correct ^H, I think Closely related to:
Thiocyanogen (CAS NO.505-14-6)
Molecular Formula: C2N2S2
Molecular Weight: 116.16g/mol
Mol File: 505-14-6.mol
Appearance: White crystals or orange liquid
Boiling point: 209.6 °C at 760 mmHg
Flash Point: 80.5 °C
Density: 1.523 g/cm3
Surface Tension: 82.2 dyne/cm
Enthalpy of Vaporization: 44.58 kJ/mol
Vapour Pressure: 0.201 mmHg at 25°C
XLogP3-AA: 1
H-Bond Donor: 0
H-Bond Acceptor: 2
Rotatable Bond Count: 1
Topological Polar Surface Area: 47.6
Heavy Atom Count: 6
Complexity: 96
Covalently-Bonded Unit Count: 1
Safety Profile
Polymerizes explosively above its melting point −2°C. When heated to decomposition it emits toxic fumes of NOx, CN− and SOx.
Specification
Thiocyanogen ,its CAS NO. is 505-14-6,the synonyms is Dicyano disulfide ; Dirhodan ; NCSSCN ; Disulfanedicarbonitrile ;
Azanylidyne-cyanodisulfanylmethane ; CHEBI:30063 ; bis[(Cyanido--C)sulfur](S--S) ; CID68160 .
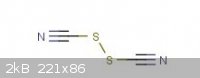
I found this on Thiocyanic acid:

[Edited on 12-5-2013 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Trithiocyanuric acid? H3C3N3S3
Perthiocyanic acid? H2C2N2S3
Perthiocyanogen? HC3N3S3
All of these are yellow compounds, insoluble in water, according to descriptions. After reading all of this, I get the impression that many people
refer to the same compound, but that different people have done experiments and different analysis, leading to different results. Maybe some people
had impure material, containing more HSCN, leading to a formula which is closer to equal amounts of H, C, S and N, hence the last formula, others may
have thiocyanate ion in their product, leading to a lower H-content.
I, however, am inclined to believe that the last one, mentioned by deltaH is the best candidate. The formation of the others does not need an
oxidizer, just acid. In my experiments, however, the presence of the nitric acid, acting as oxidizer, is essential.
The first one, H3N3C3NS, simply is formation of a trimer of HCNS.
The second one, H2N2C2S3 is a disproportionation of HCNS: 3HCNS --> HCN + H2N2C2S3, no other reagents needed.
The formation of the third one requires an oxidizer: 3HCNS --> HC3N3S3 + 2H(+) + 2e
The only issue is that perthiocyanogen only is mentioned in an old book from the early 20th century. The website, mentioned by deltaH just parrots the
original book:
http://tera-3.ul.cs.cmu.edu/NASD/01cf394d-c0ee-4090-918f-a84...
This book in turn refers to a paper from 1904. The information on internet is very sparse, apparently no one took the time and effort to investigate
this compound in more detail.
@Bezaleel: This kind of discoveries is because of my typical interests. I like to look beyond the borders of common chemistry and I have found that
especially the elements in the right upper corner of the periodic table can combine in many highly unknown and peculiar ways. I try many combinations
and this was one of the things, which I was hinted at in the past already (reaction of solid NH4SCN with solid KIO4 after ignition, formation of
yellow smoke, and also formation of yellow and orange solids in multiple experiments where thiocyanate is involved).
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Out of interest I also tried this experiment, with less HNO3 and a more or less concentrated KSCN solution (maybe a gram or so in 3ml H2O)
1.5 ml of cc HNO3 was added to this, which caused a slight heating and darkening of the mix. After about 30 seconds I notices that there was a dark
layer on top which could be diluted into the rest of the tube contents by swirling. Every time the tube was swirled a new layer of dark red
materialized upon standing. After about 2 minutes the contents were ejected quite violently with a cloud of gas and a *pang* sound.
Admittedly this was not a very well done/documented experiment and more of a small test done out of curiosity after readings woelens post. Nothing was
left in the tube to isolate, it was all ejected.
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I made a somewhat larger quantity of the yellow compound, such that I can isolate it and do some experiments with it.
I dissolved 1.5 grams of NH4SCN in appr. 10 ml of water and added appr. 5 ml of conc. HNO3, all of this in a small 50 ml erlenmeyer. The liquid turned
pale pink again. I started heating the liquid. After a while, the liquid turns darker and then it becomes turbid and starts foaming. No such violent
reaction as I had before, or as described by Mailinmypocket, but still, the foaming was so fast, that I lost about half of my liquid, it just bubbled
over the rim of the erlenmeyer 
Nevertheless, I still managed to isolate half of my liquid with quite a lot of the yellow precipitate in it. I filtered this and then rinsed two times
with distilled water, still on the filter. Finally, I put the filter on a piece of paper tissue, to absorb most of the water and then I folded the
filter and pressed it between paper tissue, to make it quite dry already and remove as much as possible of the adhering liquid. Then I let the filter
dry for one day in a warm place. After this day, I had a perfectly dry filter from which I easily could scrape the ochre/yellow powder. I now have
several 100's of mg of this compound in a perfect dry state.
A few experiments:
1) Mix with some finely powdered KClO3 and ignite the mix. The mix burns well, with a purple flame, and it is easily ignited.
2) Add a small quantity of ethylene diamine to some of the ochre/yellow solid. Everywhere, where the solid is wetted by ethylene diamine, it turns
red. The solid partly dissolves in the ethylene diamine and this highly concentrated solution is red. Add more ethylene diamine: All of the solid
quickly dissolves and an intensely yellow solution is obtained. This solution wets the glass and where the glass is wetted, the yellow color is
strongly visible. This means that the yellow color indeed is very intense.
3) Add some of the intense yellow solution to excess hydrochloric acid: The resulting liquid becomes yellow and turbid.
4) Add some of the intense yellow solution to several times its volume of water: The liquid remains deep yellow and clear.
5) Add some of the solid to concentrated NaOH: The solid dissolves with difficulty. The resulting solution is pale yellow and somewhat turbid.
6) Add some bleach: The solid dissolves more easily and the resulting liquid is clear and very pale yellow.
I'll do some more research on internet and see if I can find more information about this compound. I read already something about solubility in
organic amines and this is nicely confirmed with my experiment with ethylene diamine.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Can you smell any SO2 when ignited with KClO3?
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Woelen you yellow stuff sounds a lot like dithiocyanic acid as has already been mentioned but do you know about it interesting and complex chemistry?
Take a look at the attached paper. There are two interesting references in the paper to the preparation of potassium dithiocyanate;
A. Fleischer, Annalen, v179, p204 (1875)
A. Hantzsch & M Wolvekamp, Annalen, v331, 265 (1904)
If your material is the free acid it should contain a free cyano group it may condense with hydrogen sulphide (to give a thioamide group),
hydroxylamine to give a formamidoxime which may spontaneously cyclotise, or sodium azide and ZnCl2 to give a weird substitute tetrazole. Could be some
really weird chemistry here!
Attachment: Potassium Dithiocyanate chemistry JACS Timmons & Wittenbrook 1966.pdf (1008kB)
This file has been downloaded 709 times
The only thing is why does it require nitric acid to produce it? One reference (Cyanogen ... already give above) state that sulphuric acid on alkali
thiocyanate also works have you tried this? I don't have access to the other two paper but they sound interesting if anyone else can get them.
[Edited on 10-12-2013 by Boffis]
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I will try an experiment by heating acidified solutions of KSCN, using H2SO4 as acid. I saw in my experiments that the nitric acid acts as oxidizer,
with dilute H2SO4 there will be no oxidation. If I get the yellow compound in that case, there must be another reaction. The experiment is easy
enough, so I'll report back on this when I have done it. I'll especially check for formation of lots of HCN (flammable gas, easy to detect). In my
experiments with HNO3 the produced gas is not flammable and gives brown gas on contact with air.
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did the experiment with H2SO4 instead of HNO3. There is a large difference. Below follows the result of the experiment.
I took some a solid lump of NH4SCN. To this I added approximately 3 times its volume of 2 M H2SO4. The NH4SCN dissolves in the dilute H2SO4 and the
solution becomes pale pink, just as with HNO3.
I carefully heated this pale pink solution. On heating up, the liquid slowly turns pale yellow instead of pale pink. At the time, when the liquid is
near boiling, the color is bright yellow, a really pure yellow, like a solution of a cerium(IV) salt and also quite intense.
I kept on heating. After a minute or so, gas was produced, but at the same time the liquid started boiling. The liquid also becomes turbid and a
yellow/orange solid is produced. The gas mix is flammable, when a flame is kept near the open end of the test tube, then the flame goes into the test
tube without any sound.
On longer heating, some compact yellow/orange solid is formed. Further tests of the gas mix did not show any flammability, probably the gas mix is a
mix of HCN and a lot of water vapor. There was a peculiar smell though, but as soon as I smelt that, I stopped the experiment and left the room (maybe
I smelled the HCN, the smell was not bad or pingent, but I dared not continue the experiment). Now, I have the test tube loosely stoppered, standing
in my lab.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have just found the following excerpt in Richter's Organic chemistry vI that I think explains both the action of nitric and sulphuric acid on
thiocyanates. It would appear that fairly strong no oxidising acid liberate thiocyanic acid which then polymerises even in the cold to dithiocyanic
acid which with KOH give the sparingly soluble K salt. Boiling with acid decomposes the free acid.
Oxidising agents give Cyanogen sulphide or cyanogen pseudosulphide. The former may result from the reaction of liberated sulphur with dithiocyanic
acid. The so-called cyanogen sulphides seem rather uncertain compounds so I did an online search for them to see if anyone had subjected them to
modern investigation but found nothing "modern".
The "xanthene hydride" sound like it could do with a modern investigation too. It looks like this is from the A Fleischer reference I posted; I'll
request them and see what they have to say.
[Edited on 10-12-2013 by Boffis]
 
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I read the following and what is mentioned here may also explain what I observed:
http://carbon.atomistry.com/thiocyanic_acid.html
HSCN apparently decomposes to COS and NH3 as well when heated. So, the flammability I observed may not (only) be due to formation of HCN. COS is
flammable as well and burns with a pale blue flame. The flames I observed indeed were pale, but HCN also has a fairly pale flame as far as I remember.
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
After two days of standing, the liquid deposited more yellow material. This material has a lighter color than the material I had after the heating. It
is yellow, like the skin of a banana (without the black spots) and seems to have the same matte texture.
I decanted all liquid from the yellow material (which is yellow as well, and clear) and then I added some ethylene diamine. The material dissolves
very quickly and the resulting solution is dark olive green. In very thin layers (liquid sticking to the glass), the solution looks yellow.
As a counter experiment, I added a drop of water to my dry yellow/ochre powder from my earlier experiments and then I added a small amount of ethylene
diamine to that as well. That solution is dark red at very high concentration and is orange/yellow at lower concentration.
To both solutions I added a lot of water. The resulting solutions are yellow.
This experiment adds more questions than answers. Why the different colors? Is the result of heating with H2SO4 instead of HNO3 really different?
|
|
|
| Pages:
1
2 |