| Pages:
1
..
3
4
5
6 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Neat. I love seeing photos of copper complexes. What method(s) did you use to verify the identity of your product?
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Well Apart from the that its a complex, I don't actually know anything about it. All I can say is that it dissolves in a solution of sodium
bicarbonate, and when enough HCl is added to this mixture the deep green colour disappears and a white precipitate (presumably salicylic acid) can be
seen floating about.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Did you try dissolving the acetylsalicylic acid in bicarb before adding the metal? Also, with slight excess of the acetyl salicylic acid?
News on my synthesis:
I obtained... a deep blue oil, interestingly enough. I wish we still had our IR setup in my lab, I could check if it was actually coordinated :/
|
|
|
Semmelweis
Harmless

Posts: 10
Registered: 9-10-2013
Location: Brazil, where we get coconuts from cocopalms
Member Is Offline
Mood: A lack of 5-hydroxytryptamine
|
|
I guess a more direct approach would be to react the ASA with CuOH.
It's too weak a base to cause hydrolysis.
I make my CuOH from electrolysis. Fun compound to mess with, one easily gets copper chloride to show it's very exothermic reaction with Al.
I think I will be trying this as soon as I get more Aspirin.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Sometimes I wonder why I even bother.
ASA is quite insoluble in H<sub>2</sub>O at 20°C. If you heat to increase solubility, it will decompose. Also, your "CuOH" is
not. It's a complicated aqueous complex precipitated mess. I think you'll be hard-pressed to get them to react, and even if you do, you'll have to
wash excess ASA out of your product with an organic solvent and the leftover [CuOH] with a weak acid, such as dil. ethanoic acid (use of a strong acid
would turn your product back to ASA and a Cu<sup>2+</sup> salt). In summary, your proposed method would be just as much work, and likely
have lower yields and poor purity (inclusion of [CuOH] into precipitate during crystal growth).
A tip: <em>I've found that (in my experience)</em> it's generally a bad plan to react an insoluble species with a solution when you're
expected product is itself insoluble—it's generally slower and affords lower yields of a lower purity product than other means.
[edit] P.S. I see that you're from Brazil; any chance you could U2U me with a city name? I'm looking for someone to collect and ship a mineral
specimen.
[Edited on 16.10.13 by bfesser]
|
|
|
Semmelweis
Harmless

Posts: 10
Registered: 9-10-2013
Location: Brazil, where we get coconuts from cocopalms
Member Is Offline
Mood: A lack of 5-hydroxytryptamine
|
|
Well, I thought it could be done on ethanol, evaporate, and wash with the weak acid as you said. In fact, I have just done a test with 1 crushed
aspirin tablet, some ethanol and a little copper hydroxide, everything eyeballed in a test tube, and after shaking a lot got a color surprisingly near
that of the photo. I know, however, that for this to actually work would be against all odds.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I recently repeated my experiment with nickel carbonate but this time I used salicylic acid instead of aspirin. The nickel carbonate dissolves in the
suspension of salicylic acid producing a pale greenish liquid that would no crystallise on cooling, on evaporation (a pale green glassy residue was
obtained) or on treatment with excess alcohol. I suspended 0.5g of salicylic acid in 10ml of water and heated almost to boiling and added basic nickel
carbonate until no further effervescence occurred, filter it while hot through a small hirsh funnel to remove the excess carbonate and cooled it
slowly in an open 25cc beaker. By the following morning almost half the liquid had evaporated but no crystals had formed. Evaporation to dryness in a
shallow porcelain basin left a glassy residue which dissolved again rapidly in a few cc of water and would not crystallise even when 5ml of ethanol
(95%) were added. I conclude that the salicylate complex of Ni2+ is very soluble. It also appears identical to the product that I had obtained from
aspirin, which tend to confirm bfessor's contention that the boiling would have hydrolysed the aspirin.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Interesting. Thanks for sharing your experimental results.
|
|
|
AlChemicalLife
Harmless

Posts: 37
Registered: 18-1-2011
Member Is Offline
Mood: happy and ready for science
|
|
I was thinking of posting this in a new thread but, Since there is already a thread on this ,i didn't want to annoy people with making another thread
of the almost exact same thing , the only thing different is this starts with copper metal. But if you guys would like to take a look that would be
great, My synthesis of this compound was using Anon's video on making this Copper Aspirinate, and making it from scratch.
So A few days ago i was asked to write up an experiment which started out with the metal and ended up as a useful compound that could be used as a
pharmaceutical or in a way that could help life. So i thought and thought, and experimented with different things , searching YouTube and Google. And
i came across a few that i liked but the one that caught my eye was copper aspirinate
, A Copper compound that is used as a treatment for rheumatoid arthritis interested me, than i realized , How am i going to go from copper to the
Compound. I kept searching, and found a synthesis that i liked, that used compound's i know how to make from copper , and i went the easiest wrought
to do the following Experiment and this experiment is in the processes of being put into my website , but there are a few minor things i will change.
"From Copper metal to Pharmaceutical"
In this experiment I will start with Plain copper metal(And a Few house hold materials) , and end up with Copper aspirinate.
First we need to make a soluble copper compound that will react with Sodium Bicarbonate, usaly we could use copper sulfate, but lets say you dont have
that available,Are you out of luck?
Well , luckily for you your not out of luck, We can make another soluble copper compound that will react with Sodium bicarbonate to form Copper
carbonate, We will make Copper acetate.Than we will react the copper acetate with sodium carbonate to form copper carbonate , Than we will isolate ASA
(Acetylsalicylic acid) From aspirin, and then react the Acetylsalicylic acid with the copper carbonate to form Copper aspirinate
Making the copper acetate: Making this compound could not be easier, you will want to make up a solution with vinegar and hydrogen peroxide, you will
want to use double the amount of vinegar that use use from hydrogen peroxide ( Say you use 30mls of hydrogen peroxide , Thank you will want to use
60mls of Vinegar ). After you make this solution you will want to drop in some copper ( About 10grams should be plenty, but if you run out of copper
add more ) Now heat the solution To a slight boil and add the copper , you will notice it bubble.( NOTE: you can also just add the copper without
heating , this will take longer, but is safer ) As its bubbling you will notice the color change from clear to blue(This is NORMAL) ,the reaction is
complete when the solution is a deep blue or when you copper metal stops reacting . The Equation is here - CH3COOH+H2O2+Cu =
Cu(CH3COO)2+H2O+O2.
Now that you have the solution we will move onto making the Copper Carbonate
Making the Copper Carbonate: Making the copper carbonate is easier than make the Copper Acetate. What you want to do now is make up a Saturated
solution of Sodium bicarbonate, After you have a solution of Sodium Bicarbonate , take that solution and add it to the copper acetate solution, (You
will notice a green/blue precipitate This is our copper carbonate ) Keep adding the solution till no more precipitates out of solution. After you have
all of it precipitated out of solution , filter and wash with copious amounts of cold water to remove trace amounts of Bicarbonate that may be in your
product. Set the copper carbonate out to dry. While we wait for this to dry we now can make our Acetylsalicylic acid.
Making the Acetylsalicylic acid from aspirin : Making Acetylsalicylic acid, isn't to difficult , its actually pretty fun , So lets get started .
You will first want to start out by placing some water into a freezer to cool it down to a low temp , just above freezing.
As we are waiting for the water to cool, We can prepare our solution we will pour into the cool water.
Start by measuring about 1ml of Isopropyl alcohol per aspirin tablet used, in this experiment i used about 25 Tablets , so i will measure out 25ml's
of Isopropyl Alcohol.
Then add the Tablet, and the Isopropyl alcohol to a beaker and lightly heat do dissolve all the aspirin (DO NOT BOIL THE ALCOHOL!, Boiling it may
cause the ASA to Turn in to Salicylic acid ) After all the aspirin has dissolved,There may still be a cloudiness do to binders N' such that are not
soluble in the IPA.
Take this solution a filter it to make sure no binders are left, Then take you water out of the freezer, now take your solution with the ASA in it and
add it to the cold water.
You should see a nice fluffy white precipitate come out of solution, This is you ASA and you can take this and filter this off, while filtering wash
with copious amounts of cold water and ice cold Isopropyl alcohol.After This is done you can now dry it or recrystallize it(this isn't necessary, but
will improve your product to just make sure no binders are left) . To recrystallize just add just enough Hot Isopropyl alcohol to dissolve all the
product , Then set that out to recrystallize, putting a continues air stream helps yield. Dry this and we will move on to the next and final step to
our tour of making a pharmaceutical.
Making The Copper Aspirinate: YAY !!FINALLY!! You have survived this long and now its time to make the final product!!, this, like all the other steps
isn't to difficult.
First weigh out 9 grams of Acetylsalicylic acid and 3 grams of Copper(II)carbonate.
add the 9 grams of Acetylsalicylic acid to 75ml's of distilled water, Bring this water close to boiling. Now With Lots of stirring (Preferable with a
hotplate stirrer ) slowly add the copper carbonate, adding to much at once will result in the reaction overflowing onto the hotplate. You will note a
color change from the greenish blue copper carbonate to the Blue Copper Aspirinate. After all the carbonate has been added keep stirring and gentle
heating for about 5-10 minutes then shut off the stirring and heat and let the Copper Aspirinate settle. after its settled , if you observe bubbling,
turn back on stirring and gentle heating,this means the carbonate and Acetylsalicylic acid haven't fully reacted.
When your ready to proceed, filter the product ( Using a vacuum is highly rec emended ) At this stage your product is impure with left over Un-Reacted
Acetylsalicylic acid, add the product to a beaker , and add approximately 100ml's and stir to dissolve impurity's. Now re filter you product, it
should look MUCH cleaner , if there are anymore impurity's try and get them out by picking them out . Now Dry your product and your finished!! After
this Long experiment you deserve a coke or Pepsi (Or a beer for you fellow beer drinkers) to drink , and a pat on the back, you have made a
pharmaceutical from copper metal,aspirin , and a few other house hold products!
any questions of this are welcome !! Hope you enjoyed it.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Why do people keep insisting on reacting an insoluble copper compound with acetylsalicylic acid? Please read everything written above to understand
why this is a bad route. By the way, most of the videos I've seen for copper(II) aspirinate on YouTube have been just plain awful. You should try
doing a little proper research before blindly following anything you see on YouTube. You'll find that many of the videos, if not all, were made after
this topic was opened. Sadly, none of the authors seem to have read through this or understand what they're actually doing.
[edit] If you put this on your website, a link to Science Madness would be appreciated.
[Edited on 8.12.13 by bfesser]
|
|
|
AlChemicalLife
Harmless

Posts: 37
Registered: 18-1-2011
Member Is Offline
Mood: happy and ready for science
|
|
I just wrote that experiment up for fun, weather or not it was effective or efficient wasn't on my mind. And when i get done with proofing and taking
picture ill post it on my website and put a link to this thread , and it will be noted this is not the most efficient or effective way to make it, but
it is fun and i enjoyed it  . .
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
First off, feels good to be done with the semester and back on ScienceMadness! Back to my aspirinate fun!
Second,
Quote: Originally posted by AlChemicalLife  |
Bring this water close to boiling. Now With Lots of stirring (Preferable with a hotplate stirrer ) slowly add the copper carbonate, adding to much at
once will result in the reaction overflowing onto the hotplate... |
If you heat it, you run the risk of decomposition, likely forming some salicylic acid. Why not skip the carbonate synthesis step and make it directly
from copper acetate??
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Just found this gem:
<strong>Hybrid (Electrochemical-Chemical) Single Crystal Synthesis of Copper Aspirinate Starting from an Aspirin Tablet: an Undergraduate
Bioinorganic Experiment.</strong>
A. Pérez-Benítez, M.A. Méndez-Rojas, S. Bernès, E. González-Vergara
<em>Chemical Education Journal (CEJ)</em>, Vol. 11, No. 2 /Registration No. 11-8 /Received January 23, 2008
<a href="http://chem.sci.utsunomiya-u.ac.jp/v11n2/PerezBenitez/PEREZ_BODY.html"
target="_blank">http://chem.sci.utsunomiya-u.ac.jp/v11n2/PerezBenitez/PEREZ_BODY.html</a> <img src="../scipics/_ext.png" />
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
That is awesome! ! ! Anyone try it yet?
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I replicated the experiment shortly after that post, substituting 0.9 mm pencil lead, and got very poor results. I did get some product, but the
crystals are much smaller than claimed by the original authors—I didn't bother to recover them.
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
Here is a video I made on the synthesis of copper aspirinate. Hope you enjoy it.
When the police come
\( * O * )/ ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nice video, but it's incorrect to refer to the copper aspirinate as a ligand. The aspirinate ion acts as a ligand to the copper, but the whole thing
isn't a ligand.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Nice work, kid. You've made the only decent video I've seen of copper(II) aspirinate, so far! I'd recommend correcting the narration to refer to it
as a "copper chelate" (<a href="https://ssl.gstatic.com/dictionary/static/sounds/de/0/chelate.mp3"
target="_blank">/ˈkēˌlāt/</a> <img src="../scipics/_ext.png" /> or "copper coordination complex" (<a href="https://ssl.gstatic.com/dictionary/static/sounds/de/0/coordination.mp3"
target="_blank">/kōˌôrdnˈāSHən/</a> <img src="../scipics/_ext.png" /> or "copper coordination complex" (<a href="https://ssl.gstatic.com/dictionary/static/sounds/de/0/coordination.mp3"
target="_blank">/kōˌôrdnˈāSHən/</a> <img src="../scipics/_ext.png" /> , and putting a visual in the black screen part near the end. , and putting a visual in the black screen part near the end.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Copper imidazole complex
Nice video man! Since we are giving pointers (and this is what I tell my students), you should give molar quantities rather than grams of this plus
grams of that. It is much more scientific and less "cooking" style.
Anyways, back to the fun of copper complexes since I will have free time this weekend!
First things first, my imidazole complex of copper seemed to work (seemed since I have a waxy, dark blue product now, and no access to an FTIR). I
guess if you guys want to try and confirm this little experiment I ran, I'll write up a little blurb. I don't have any idea for purifying so far,
maybe you guys will have ideas.
0.232 g (3.4 mmol) of imidazole was dissolved in ~100% ethanol. To this solution, 0.72 g (0.85 mmol) of copper (II) aspirinate was added. The cloudy
blue solution was stirred for an hour.
And this is where my notes went to crap and it has been awhile since I actually performed the experiment, so I am not positive if I did anything after
that step. All I know is that I let it air dry for several days and got a waxy, blue solid.
Ideas/comments/etc. are encouraged here. I am in the process of remaking some copper aspirinate to redo it.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Copper (II) Benzoate Synthesis
Also, I noticed that no one has a write up for copper (II) benzoate. I like my method which uses both organic and inorganic routes to the product. Not
really scientific writing, but eh, I've been grading papers all day.
1) Benzoic acid from toluene. I love this reaction way more than I should, plus it is a great beginner organic reaction. No write up here (Someone
really should put one in the "Pre-Pub" section though), just a nice video of the synthesis: benzoic acid
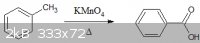
2) Next, we can take our fresh benzoic acid and deprotonate it with sodium hydroxide in water. The important part for this step is to make sure that
the benzoic acid is in excess and can be filtered away before the next step. This ensures that there is no leftover sodium hydroxide in the solution,
which will inadvertently react with copper sulfate.

3) Lastly, to your filtrate from step 2, add half an equivalence of copper (II) sulfate pentahydrate. Immediately, the product, copper (II) benzoate,
will crash out of the solution and can be obtained by filtration and rinsed thoroughly with water. This yields fine, pale blue crystals.

Simple synthesis, really, and emphasizes a lot of good chemistry and techniques.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I suspect your structure for copper(II) benzoate is inaccurate. Isn't it similar to the acetate, actually being dimeric?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Didn't think about it, but here is the wiki answer:
http://en.wikipedia.org/wiki/Copper_benzoate
"Copper(II) benzoates exists in at least two structural forms, depending on the degree of hydration."
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Nice route but, the deprotination seems unnecessary IMO, as you form potassium benzoate in the first step, which then you acidify and then
deprotinate... The solubility between the two is negliable too (sodium benzoate 62.9 g/100 ml water, potassium benzoate 65 g/100 mL- wiki) I am I
missing something or..?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The structure depicted on the Wikipedia page is most certainly completely wrong. Interestingly, the wiki article itself claims a different structure
in its Structure section. Anyway, if you follow the references it gives, you can see that the various copper(II) benzoates all are coordination
compounds, just like the copper carboxylates generally are. The benzoate ligand acts as bridging ligands between two copper atoms. See Acta
Cryst. 1992, B48, 253-261 (DOI:10.1107/S0108768191012697, attached) for the structure of some
Cu2(BzO)4L2 compounds.
I don't have the access to DOI: 10.1143/JPSJ.18.117 where the structure of copper benzoate trihydrate is reported, but then again neither do you seem
to know which copper(II) benzoate did you obtain.
Attachment: ActCryst_B48_253_261.pdf (278kB)
This file has been downloaded 679 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Quote: Originally posted by HeYBrO  |
Nice route but, the deprotination seems unnecessary IMO, as you form potassium benzoate in the first step, which then you acidify and then
deprotinate... The solubility between the two is negliable too (sodium benzoate 62.9 g/100 ml water, potassium benzoate 65 g/100 mL- wiki) I am I
missing something or..? |
I should have clarified, while it is cool and dandy to make copper benzoate, I do not need a huge surplus of it. So I made the benzoic acid, isolated
it, and used only the amount I wanted for the copper benzoate synthesis.
Thanks Nicodem for the correct structure, it's been quite some time since I've played with inorganic. Just goes to show you how "reliable" wiki is.
| Quote: |
but then again neither do you seem to know which copper(II) benzoate did you obtain.
|
Not even the slightest clue 
-Sarge
|
|
|
| Pages:
1
..
3
4
5
6 |