| Pages:
1
2
3 |
questions
Hazard to Others
  
Posts: 103
Registered: 12-2-2011
Location: Australia
Member Is Offline
Mood: curious
|
|
making sodium
I loved your method of making sodium metal given below;
http://www.sciencemadness.org/talk/viewthread.php?tid=9797
I am thinking of using this method to make sodium metal and was wondering if any strong and noticeble fumes and odors were released durring the
process?
[Edited on 5-4-2011 by Polverone]
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
A number of people have asked me questions about sodium, and also UV chlorination in the other thread.
I no longer recommend the method in this post. The Sodium chapter in my book gives a thorough explanation of a much simpler setup. UV chlorination is
also explained in the CCl4 chapter, I havent time to repeat all that here.
If you want to see it its now on google books. Search for the title in the Book thread. The page restriction on google books can easily be got around
by going to another computer.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Someone asked me for a link to the Sodium chapter I referred to. Here its is.
http://books.google.com.au/books?id=VqosZeMjNjEC&pg=PA14...
|
|
|
ScienceSquirrel
|
Thread Pruned
13-3-2012 at 05:40 |
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
The attached document describes the construction of a Castner cell for producing sodium 
Attachment: ExperimentalElectrochemistryXI.pdf (718kB)
This file has been downloaded 1895 times
Chemistry is our Covalent Bond
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
This is a chapter
from Experimental Electrochemistry, by Nevil Monroe Hopkins, 1905. Interesting that this was almost a full century after it was first done by
Davy.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I might be building a Castner cell soon, and having not read this entire thread yet, I'm not sure if the insolating plug ( for the cathode)
problem was completely solved.
In the first post, len1 used clay, which deteriorated after a while.
My idea is to use a light bulb screw-plug (or whatever it's called.) The idea would be to brake a bulb (preferably a dead one), wire the PSU to the
wires that go into the bulb. Then solder cathode to the very bottom of the "screw-plug". So it screws in upside down, into the bottom of the cell.
The metal jacket terminal is directly connected (screwed) to the nickel cell casing which serves as the anode.
Some problems are:
•Light bulb terminals are made out of aluminum (at least the ones I've tested), which of course will react with the NaOH melt. Perhaps it can be
coated in PTFE tape, and connected to the anode with solder too.
•I have not tested whether NaOH reacts with the black insolating ceramic yet - maybe it will.
•The wires going into the bulb are very thin, and will certainly over-heat at more than ~5 amps I'll bet. It's quite cramped inside the bottom of
the bulb and very hard to solder a new thicker wire - with patience, I could do it I'm sure.
Other than that, it seems like it wouldn't be very hard to make this cell. I can't wait!
Here's a crappy illustration of the idea for those still confused.
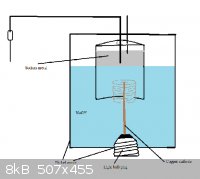
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Just an idea like that, you could lower the cathode socket a lot in a tube which would not be heated, this way, Solid NaOH could act as a plug, as
such the clay would last almost indefinitely.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
PTFE will degrade in molten NaOH.
The solid NaOH plug is a good idea.
|
|
|
The Garbage Guardian
Harmless

Posts: 2
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
This is really late, but I have some questions that will hopefully be answered?:
1. Where did you obtain the larger metal parts like the cell wall and the collector? I checked local hardware stores, but they are all too small.
2. How were you able to tell if they and the stainless steel pots were galvanized/coated?
3. Is current for the cathode passed through the stub sticking out the bottom of the stem?
"Jerma and I relentlessly defend the garbage from all foreign invaders, utilizing techniques such as the people's elbow." -STAR_
|
|
|
UncleJoe1985
Hazard to Self
 
Posts: 88
Registered: 30-9-2008
Location: Sunnyvale, CA
Member Is Offline
Mood: No Mood
|
|
I was in the process of building my own cell after watching several Youtube videos, until I came across this excellent guide that makes me reconsider
my cell design.
1. Is having the cathode at the bottom necessary? I know the design from 150 years ago did it that way to prevent bridging and that len1 is clearly in
favor of that. The most successful cell I saw on YouTube also did it that way. I'm trying to make my cell from very thin titanium, which makes it very difficult to
weld, so having a hole on the bottom seems a bad idea. Is it possible instead to passivate the top part of the anode so that it does not cause that
awful bridging and short circuiting? What coating can withstand NaOH and not get reduced by the electron flow?
2. Is titanium a good cell body material compared to steel or nickel? According to this study, the erosion rate increases rapidly with temperature.
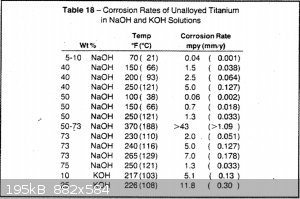
But even 1mm/year is pretty good - that's 44 kg at the 5g/hour rate of Nux's cell on Youtube. And this is for aqueous solutions, which is more
damaging to the oxide layer than non-aqueous. I heard this from other people who constructed their cell using aluminum.
Here's a picture of my cell so far. The tube is a Ticon Industries perforated punch tube for mufflers. Also, I plan on using immersion heating with
ceramic heater cartridges. They will be installed in nickel plated aluminum tubes.

|
|
|
| Pages:
1
2
3 |