(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
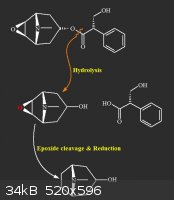
Epoxide reduction
Hi,
I have access to a considerable source of scopolamine (around 10 gr)
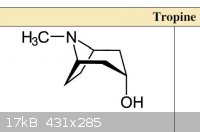
I'm thinking of the following series of reactions to make tropine.
my question is how to get rid of that epoxide, after hydrolysis ?
there's a hydroxyl group there, if matters.
TnX
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Might be easier to make Tropinone outright from simple building blocks, then reduce it to Tropine.
http://en.wikipedia.org/wiki/Tropinone
|
|
|
kch
Harmless

Posts: 26
Registered: 7-7-2013
Member Is Offline
Mood: No Mood
|
|
It's going to be very difficult to do this for two reasons. First, you have to protect your existing hydroxyl group. Next, your best product from the
epoxide is basically going to be a glycol. That isn't really feasible to work with if you don't have a hydrogenator or an equivalent setup.
|
|
|
Mercedesbenzene
Hazard to Self
 
Posts: 64
Registered: 4-9-2011
Location: BC
Member Is Offline
Mood: Sub Zero
|
|
I didn't find any papers on what you are trying to do, but by looking at it I can make a hypothesis on what might work. First I would hit scopolamine
with NaBH4. This should eliminate the epoxide leaving an alcohol and it is not powerful enough to reduce the ester.
The next step is to take the alcohol to an alkane. The best method here might be to use tosyl chloride then sodium borohydride again.
Finally cleave the ester with base.
This might give you tropine. Best way to find out is to try it!
|
|
|