(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Why This Reaction Doesn't Happen ???
I know this question is a general one, but the answer will surely help me understand basic things in reactions grasping.
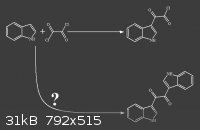
I found in shulgin's book that indole reaction with Oxalyl chloride, yields 2-(1H-indol-3-yl)-2-oxoacetyl chloride.
(attached image)
my question is NOT : the second reaction happens or not ?
I know it doesn't happen.
my question is : why the second reaction doesn't happen ?
cause there's a chlorine there and there are other indoles with intact C-3 carbon.
maybe my question is a booby one, but it's just what sparkled out of my curiosity.
TnX
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
From TIHKAL:
| Quote: |
To a well stirred solution of 10 g indole in 150 mL anhydrous Et2O there was added, dropwise over the course of 30 min, a solution of 11 g oxalyl
chloride in 150 mL anhydrous Et2O. Stirring was continued for an additional 15 min during which time there was the separation of indol-3-ylglyoxyl
chloride as a yellow crystalline solid. This intermediate was removed by filtration and washed with Et2O. It deteriorates at a significant rate at
room temperature, and should be used as soon as possible after preparation.
|
Two main reasons why the second reaction only happens on negligible scale: Is the indole in excess? No. Is the indol-3-ylglyoxyl chloride soluble in
the reaction mixture? No.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
In the procedure from TIHKAL, indole is kept in excess of oxallyl chloride by adding a solution of oxallyl chloride to a solution of indole, slowly.
Furthermore, the product, indol-3-ylglyoxyl chloride precipitates as it is formed (as far as I can tell from the procedure), so it cannot appreciably
react further with more indole.
If the procedure were reversed, adding solution of indole to a solution of oxallyl chloride, the symmetric product you wonder about would probably be
the major product. What would that be called, by the way? Bis-indol-3-ylglyoxyl?
Are you sure the bis-indole product doesn't form AT ALL? I wouldn't be surprised if it did in small amounts as a side reaction.
Edit: Dang, Kristoff beat me to it as I was typing... Oh well
[Edited on 29-7-2013 by Crowfjord]
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
maybe it very little traces of it forms.
cause shulgin says the yield after making the corresponding amide is 79%.
so can't be sure whether the first reaction or amidation causes this yield drop.
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|