(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Amine Alkylation Question
Hi all.
I'm not very experienced with practical organic synthesis.
I know :
R-X + Amine ---------------> RNH2 and/or R2NH and/or R3N and/or
R4N+X-
I want to know if the number of carbons of R in R-X would affect the products proportions ?
Example :
how do you predict whether the salt is formed or not in the following reaction :
C20H?Br + R2NH ----------> ???????
If there are any review papers on the subject of "Amine alkylation", I would get my answer by comparing the products of the reactions.
Thanks in advance
(Brain)2NH
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
The short answer? No. The long answer? Yes. It gets complicated when you start mucking about with the carbon chains, turning them into bulkier
groups and whatnot. <a href="https://en.wikipedia.org/wiki/Steric_effects" target="_blank">Steric effects</a> <img
src="../scipics/_wiki.png" /> will come into play, and the <a href="https://en.wikipedia.org/wiki/Chemical_kinetics"
target="_blank">kinetics</a> <img src="../scipics/_wiki.png" /> of the reaction will be affected. The more substituted the <a
href="http://en.wikipedia.org/wiki/Amine#Classes_of_amines" target="_blank">amine</a> <img src="../scipics/_wiki.png" />, the slower
the <a href="http://en.wikipedia.org/wiki/Nucleophilic_substitution" target="_blank">reaction</a> <img src="../scipics/_wiki.png" />
is expected to progress (rxn. rate <a href="http://dl.clackamas.cc.or.us/ch106-03/clasific.htm"
target="_blank">1°>2°>3°</a> <img src="../scipics/_ext.png" /> . Just imagine the difference in effort between swinging one bat (fig. 1), versus swinging 3 bats (fig. 2). Also, the
product is not a <a href="https://en.wikipedia.org/wiki/Salt_(chemistry)" target="_blank">salt</a> <img src="../scipics/_wiki.png"
/>. . Just imagine the difference in effort between swinging one bat (fig. 1), versus swinging 3 bats (fig. 2). Also, the
product is not a <a href="https://en.wikipedia.org/wiki/Salt_(chemistry)" target="_blank">salt</a> <img src="../scipics/_wiki.png"
/>.
See: <a href="http://en.wikipedia.org/wiki/Amine_alkylation" target="_blank">Amine alkylation</a> <img src="../scipics/_wiki.png" />
C<sub>20</sub>H<sub>?</sub>Br would be C<sub>20</sub>H<sub>41</sub>Br, by
C<sub>n</sub>H<sub>2n+2</sub>, assuming a <a href="https://en.wikipedia.org/wiki/Alkane#Structure_classification"
target="_blank">linear alkane</a> <img src="../scipics/_wiki.png" />.
<a href="http://www.homestarrunner.com/sbemail64.html" target="_blank"><img src="http://secxtanx.com/dump/cantmoveface/EatingBatteries.jpg"
width="200" valign="top" /></a> <img src="../scipics/_ext.png" />
Hope this helps. By the way, welcome to ScienceMadness! Not a bad first post. I appreciate the use of subscript. 
[Edited on 7/21/13 by bfesser]
|
|
|
Nicodem
|
Thread Moved
21-7-2013 at 05:40 |
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Recently I tried to alkylate a tertiary amine (BnNR2) with Ethyl Iodide. After several weeks at room temperature, and occasional-periods of refluxing,
the reaction had barely progressed. I then gassed it with MeBr and it was all converted after leaving at room temperature overnight. So even with
something as "small" as ethyl iodide, the rate of the reaction giving the ammonium salt can be negligible.
When you have large alkyl groups as part of your alkylating agent, you can be reasonably sure that there will be no ammonium salt produced, even under
forcing conditions. Of course this depends upon the nature of your amine and alkylating agent, but if you make a model of the reactants, it should
become obvious as to whether or not the spacial arrangement of the atoms will hinder their combination.
Unless you are using a tertiary amine, and attempting to form the salt of that, you will need a base to aid the alkylation, since it the reacting
amine may function as a base. For practical purposes, triethylamine and tripropylamine are often used as non nucleophillic bases, which should give
you an idea of the reasonably small tolerance of alkyl-substituted amines towards being shielded from reactive alkylating amines due to the bulk of
the appended alkyl groups - three ethyl groups usually being sufficient to prevent further alkyation; at least withing a reasonable time-scale, by
which time the desired alkylation will have proceeded, and the amine usually precipitated as the hydrohalide.
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
I want to be sure that no salt is formed in this reaction :
bulkyR-halide + Small 2o amine -> bulkyR,R',R"N
Small 2oAmine : Dimethyl, Diethyl, EthylMethyl
My bulky-R : a hydrocarbon of 10 carbons.
it would be no problem if NH4Halide salt is formed.
but i don't want any R containing salt to be produced. (like NR4Halide)
any guidance or tips to achieve this goal ?
thanks to all who care.
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Your alkyl bromide may be totally unreactive. Why won't say what it is? No one can help you with generalities because not all alkyl bromides will act
as alkylating agents. Does it contain 10 or 20 carbons? You have stated each.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
So you're trying to synthesize <em>N,N</em>-dimethyldecylamine (or analogues)? Are you planning on preparing the
<em>N</em>-oxide? Unfortunately, I've been unable to find a literature procedure for the synthesis of your target compound(s). Here's
what I've found:
<strong><a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8168"
target="_blank"><em>N,N</em>-dimethyl-1-dodecanamine</a></strong> <img src="../scipics/_ext.png" /> (PubChem)
C<sub>12</sub>H<sub>27</sub>N
CAS № 1120-24-7
<strong><a href="http://orgsyn.org/orgsyn/default.asp?formgroup=basenpe_form_group&dataaction=db&dbname=orgsyn"
target="_blank"><em>N,N</em>-dimethyldodecylamine oxide</a></strong> <img src="../scipics/_ext.png" />
<em>Organic Syntheses</em>, Vol. 50, p. 56 (1970); Coll. Vol. 6, p.501 (1988).
<strong><a href="http://webbook.nist.gov/cgi/cbook.cgi?ID=1120-24-7&Units=SI" target="_blank">1-Decanamine,
<em>N,N</em>-dimethyl-</a></strong> <img src="../scipics/_ext.png" /> (NIST)
<a href="http://scholar.google.com/scholar?hl=en&q=dimethyldecylamine" target="_blank">Google Scholar Search for
"dimethyldecylamine"…</a> <img src="../scipics/_ext.png" />
<a href="http://www.chemicalland21.com/specialtychem/perchem/DIMETHYLDECYLAMINE.htm" target="_blank"> | Quote: | <strong><a href="http://www.chemicalland21.com/specialtychem/perchem/DIMETHYLDECYLAMINE.htm"
target="_blank">DIMETHYLDECYLAMINE</a></strong> <img src="../scipics/_ext.png" />
This compound (tertiary Amine) is used as an intermediate for the manufacture of quaternary ammonium compounds, amine oxide and betaine surfactants
for personal care, and institutional use. It is used as a corrosion inhibitor and acid-stable emulsifier. |
</a>[edit] <strong>sonogashira</strong> is correct. Check out <strong>Table 1</strong> on the
<strong><a href="http://en.wikipedia.org/wiki/Nucleophilic_aliphatic_substitution" target="_blank">Nucleophilic
substitution</a></strong> <img src="../scipics/_wiki.png" /> article I provided earlier.
[Edited on 7/21/13 by bfesser]
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by sonogashira  | | Your alkyl bromide may be totally unreactive. Why won't say what it is? No one can help you with generalities because not all alkyl bromides will act
as alkylating agents. |
R is an indole.
my RX is 3-(2-BROMOETHYL)INDOLE
CAS# : 3389-21-7
2oamine : Dimethylamine or Diethylamine
Thanks
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Your proposed reaction:
<a href="http://www.evershinechem.com/3389-21-7-p-161.html" target="_blank"><img
src="http://www.evershinechem.com/uploadfile/product/3389-21-7.gif" valign="middle" /></a> <img src="../scipics/_ext.png" valign="top"
/> + <a href="http://en.wikipedia.org/wiki/Dimethylamine" target="_blank">(CH<sub>3</sub> <sub>2</sub>NH</a> <img src="../scipics/_wiki.png" /> → <a href="http://en.wikipedia.org/wiki/Dimethyltryptamine" target="_blank"><img
src="http://upload.wikimedia.org/wikipedia/commons/thumb/8/88/DMT.svg/200px-DMT.svg.png" valign="middle" width="120" /></a> <img
src="../scipics/_wiki.png" valign="top" /> <sub>2</sub>NH</a> <img src="../scipics/_wiki.png" /> → <a href="http://en.wikipedia.org/wiki/Dimethyltryptamine" target="_blank"><img
src="http://upload.wikimedia.org/wikipedia/commons/thumb/8/88/DMT.svg/200px-DMT.svg.png" valign="middle" width="120" /></a> <img
src="../scipics/_wiki.png" valign="top" />
(Ambiguity will only land this thread in <strong>Detritus</strong>.)
|
|
|
bfesser
|
Thread Moved
21-7-2013 at 09:46 |
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
.....find this book and learn the answers.....solo
Concerning Amines
(Their Properties, Preparations and Reactions)
David Gingsburg
1967
.....read, alkylation of amines Page 69
[Edited on 21-7-2013 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
It will work, but I doubt that ammonium salt could be avoided with dimethylamine. With diethylamine one could certainly avoid it, but it will depend
upon the CAS number of your intended solvent.
Attachment: Liebigs Ann. Chem., 1935, 520, (01), 019-030.pdf (544kB)
This file has been downloaded 442 times
[Edited on 21-7-2013 by sonogashira]
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by sonogashira  | I doubt that ammonium salt could be avoided with dimethylamine. With diethylamine one could certainly avoid it
[Edited on 21-7-2013 by sonogashira] |
So the formation of salt depends more on the secondary amine than Alkyl-Halide ?
Is it what you meant or I'm misunderstanding you purpose ?
thanks again
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I am saying only that diethyltryptamine will react with your alkylating agent reasonably slowly, and dimethytryptamine will react with it quite
rapidly, as you can see in that paper.
Why talk of generalities of reactivities of secondary amines with alkyl halides?
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
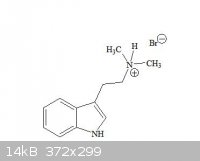
The paper is in german but based on pics I think it said the attached image is produced in my proposed reaction, Right ?
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Yes, it is produced along with the tertiary amine.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>(Brain)2NH</strong>, your reaction would be difficult to control, would result in a mixture of products, and uses an expensive
substrate. This is neither an efficient nor a cost effective route to the target molecule. If you're genuinely interested in the theory, I recommend
that you study the literature to get a better handle on the chemistry involved. If you just want someone to tell you how to synthesize your target,
try searching Erowid, Hive, Rhodium, etc.
[Edited on 7/21/13 by bfesser]
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Sorry, I'm asking too many questions on this thread.
But only this last unresolved question:
If tertiary amine reacts with a released HBr molecule, then this one is made.
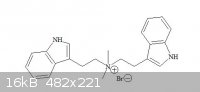
how to predict whether this salt (attached image) is more prone to be formed or the previous image ?
my guess (raw guess) is this one is more possible.
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
If you ignore the excess of dimethylamine that you have in solution, then certainly it will be formed in preference. What does it matter anyway? Are
you interested in studying the mechanism? The reaction will work, and quaternary ammonium bromides are easy to remove.
[Edited on 22-7-2013 by sonogashira]
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Would addition of a strong amine base, like diisopropylethylamine mitigate the formation of the quaternary amine in this particular reaction?
Hunig's base is the only non-nucleophilic amine that I can think of for this purpose... Sterically hindered pyridines like 2,6-lutidine are probably
too acidic to work for this purpose, am I right?
|
|
|
Prometheus23
Hazard to Self
 
Posts: 62
Registered: 6-6-2012
Member Is Offline
Mood: No Mood
|
|
I know this probably isn't a very practical alternative given that your tertiary amine would be easily separated from the quaternary ammonium salt
by-product, but I've been working with dodecanethiol lately so it came to mind.
If you are concerned about overalkylation to the quaternary ammonium salt then one possibility would be to use trimethylamine (or
N,N-diethylmethylamine) instead of dimethylamine (or diethylamine). This would of course lead to the quaternary ammonium salt exclusively. However
heating this product with sodium dodecanethiolate (formed in situ from dodecanethiol and a strong base) in DMSO will produce the N-demethylated
product. If trimethylamine is used then the final product would be N,N-dimethyltryptamine, and if N,N-diethylmethylamine is used then it would be
N,N-diethyltryptamine.
Again, there are obviously simpler methods if you already have dimethylamine or diethylamine. But I've been experimenting with dodecanethiol for
N-demethylations as well as O-demethylations of aryl methyl ethers recently.
|
|
|