Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Pine resin derivatives
I created this thread as pine resin seems like easy available material which could be used as precursor to some interesting chemicals. Since I don't
have distillation set up, I tried to extract pinenes with ethanol/isopropanol mixture. Which wasn't sucessful but did produce some interesting
results. I'm certainly going to do more experiments with it.
Procedure:
Alcohol mixture was saturated with pine resin and filtered, giving a golden yellow solution which smelled strongly of resin. About 5mL was put into
the test tube.

On addition of 5mL water precipitate started forming.

Small droplet of something was floating on solution.

Addition of 5mL more didn't cause more precipitate to form or dissolve already precipitated stuff.

Solution was decanted with small amount of sticky yellow stuff.

Solution above it was filtered since it was cloudy through coffee filter, almost nothing was caught on the filter. Here is filtered solution.

Yellow stuff was put on paper from test tube, it was a lot less sticky than actual ethanolic extract. Seemed like handling crystallized honey mixed
with water.

Close-up of yellow precipitate.

I have thought that I'll get a small layer of pinenes floating at the top but that was not the case. Resin was dried a little bit but still there
should be at least some of them. Different components or mixtures of them were separated though:
1) milky white solution (need to perform colloid test)
2) yellow sticky precipitate
Sticky precipitate doesn't react at all with sodium bicarbonate solution, which either means it's not resin acid (90% of resin acids is abietic acid)
or it's actually weaker acid than carbonic. Or maybe it needs more time to react.
Now few questions are:
1) What is the milky white stuff that can't be filtered?
2) What is the yellow precipitate?
3) Where did pinenes go?
I would like to hear some thoughts on this. If this is wrong section please move the thread, but I think I'll post more experiments on this topic in
the future.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Chemistry
The resin produced by most plants is a viscous liquid, composed mainly of volatile fluid terpenes, with lesser components of dissolved non-volatile
solids which make resin thick and sticky. The most common terpenes in resin are the bicyclic terpenes alpha-pinene, beta-pinene, delta-3 carene and
sabinene, the monocyclic terpenes limonene and terpinolene, and smaller amounts of the tricyclic sesquiterpenes, longifolene, caryophyllene and
delta-cadinene. Some resins also contain a high proportion of resin acids. The individual components of resin can be separated by fractional
distillation.
A few plants produce resins with different compositions, most notably Jeffrey Pine and Gray Pine, the volatile components of which are largely pure
n-heptane with little or no terpenes. The exceptional purity of the n-heptane distilled from Jeffrey Pine resin, unmixed with other isomers of
heptane, led to its being used as the defining zero point on the octane rating scale of petrol quality. Because heptane is highly flammable,
distillation of resins containing it is very dangerous. Some resin distilleries in California exploded because they mistook Jeffrey Pine for the
similar but terpene-producing Ponderosa Pine. At the time the two pines were considered to be the same species of pine; they were only classified as
separate species in 1853.
Some resins when soft are known as 'oleoresins', and when containing benzoic acid or cinnamic acid they are called balsams. Oleoresins are naturally
occurring mixtures of an oil and a resin; they can be extracted from various plants. Other resinous products in their natural condition are a mix with
gum or mucilaginous substances and known as gum resins. Many compound resins have distinct and characteristic odors, from their admixture with
essential oils.
Certain resins are obtained in a fossilized condition, amber being the most notable instance of this class; African copal and the kauri gum of New
Zealand are also procured in a semi-fossil condition.
http://en.wikipedia.org/wiki/Resin
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Thanks for the information solo.
Also some interesting things I have found:
http://en.wikipedia.org/wiki/Resin_acid
| Quote: |
Production in tall oil (chemical pulping byproduct) See also: Tall oil The commercial manufacture of wood pulp grade chemical cellulose using the
kraft chemical pulping processes releases resin acids. The Kraft process is conducted under strongly basic conditions of sodium hydroxide, sodium
sulfide and sodium hydrosulfide, which neutralizes these resin acids, converting them to their respective sodium salts, sodium abietate,
((CH3)4C15H17COONa) sodium pimarate ((CH3)3(CH2)C15H23COONa) and so on. In this form, the sodium salts are insoluble and, being of lower
density than the spent pulping process liquor, float to the surface of storage vessels during the process of concentration, as a somewhat gelatinous
pasty fluid called kraft soap, or resin soap.[1] Kraft soap can be reneutralized with sulfuric acid to restore the acidic forms abietic
acid, palmiric acid, and related resin acid components. This refined mixture is called tall oil. Other major components include fatty acids and
unsaponifiable sterols. Resin acids, because of the same protectant nature they provide in the trees where they originate, also impose toxic
implications on the effluent treatment facilities in pulp manufacturing plants. Furthermore, any residual resin acids that pass the treatment
facilities add toxicity to the stream discharged to the receiving waters. |
A part of the acids might have actually slowly reacted with resin acids forming sodium abietate mainly. That could explain the yellow stuff floating
on the top of test tube.
http://en.wikipedia.org/wiki/Resin_soap
| Quote: |
Resin soap is a mix of salts (usually sodium) of resin acids (usually mainly abietic acid). It is a yellow gelatinous pasty soap with use in bleaching
and cleaning and as a compound of some varnishes. It also finds use in rubber industry. Resin soap is made by reacting resin acids in wood with
sodium hydroxide, as a byproduct of the Kraft process for manufacturing wood pulp. It is also called Kraft soap. Acidification of the resin soap
produces tall oil. Pine soap is refined from resin soap via tall oil by acidification, refining and resaponification. |
"Tall oil"
http://en.wikipedia.org/wiki/Tall_oil
| Quote: |
See also: Resin acid
The composition of crude tall oil varies a lot, depending on the wood furnish used. A common quality measure for tall oil is acid number. With pure
pines it is possible to have acid numbers in the range 160 - 165, while mills using a mix of softwoods and hardwoods in the furnish might give acid
numbers in the range of 125 - 135.[2]
Normally crude tall oil contains rosins (which contains resin acids (mainly abietic acid and its isomers), fatty acids (mainly palmitic acid, oleic
acid and linoleic acid) and fatty alcohols), unsaponifiable sterols (5-10%), some sterols, and other alkyl hydrocarbon derivates.[3]
By fractional distillation tall oil rosin is obtained, with rosin content reduced to 10-35%. By further reduction of the rosin content to 1-10%, tall
oil fatty acid (TOFA) can be obtained, which is cheap, consists mostly of oleic acid, and is a source of volatile fatty acids. |
[Edited on 3-7-2013 by Random]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Update:
Test tube with milky solution is still cloudy but a fine precipitate formed at the bottom, very small amount though. I guess the yellow stuff were
resin acids but white precipitate isn't, there is too small amount of it per amount of resin used. I'll try to examine some of its properties, maybe
some more will decant with time.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
This chapter from Raphael Ikan's Natural Products book might be of interest to you.
Attachment: Rosin.pdf (141kB)
This file has been downloaded 2617 times
|
|
|
paw_20
Harmless

Posts: 32
Registered: 14-8-2012
Location: United States
Member Is Offline
Mood: Curious
|
|
That little tidbit on n-heptane derived from the resin of the Jeffrey Pine is pretty cool solo, as so many substances found in plants are formed of 5
carbon isoterpene units. Interesting.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Thanks for info Paddywhacker, I would certainly love to reduce the acid but evolution of H2S discourages me. It was interesting read though.
On another note I have decanted the small amount of white precipitate and tried to dissolve it again in alcohol, where it yielded colorless solution.
That means it does not contribute to yellow resin solution color, which I guess it contains about 90% resin acids. But if resin acids were actually
among yellow precipitate, what is the white precipitate? I guess it doesn't make more than 5% of whole resin by weight, maybe even less.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
If the resin is a mixture of acid and neutral ingredients then you may be able to separate them by extracting the acid into aqueous alkali. Maybe you
can prepare salts such as the calcium salt.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by Paddywhacker  | If the resin is a mixture of acid and neutral ingredients then you may be able to separate them by extracting the acid into aqueous alkali. Maybe you
can prepare salts such as the calcium salt.
|
You think calcium salt would be soluble?
I'll try mixing powdered resin with Ca(OH)2 and filter then acidify.
Edit:
I tried mixing pine resin powder with NaHCO3 but no reaction was seen. I'll probably need some hydroxide for this.
[Edited on 12-7-2013 by Random]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
White stuff is colloid, you can try to add some salt into it hoping to precipitate it.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
No, but people have pyrolyzed calcium salts alone, with NaOH or with sodium acetate to produce various derivatives. The sodium salt is more likely to
be soluble, then acid (say, citric acid) would precipitate the rosin acid. I don't know, it's all speculative, but photographs are always
entertaining and informative.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
papaya, never thought of this, I'm going to do it next time because I threw this sample away..it was a very small amount though and it precipitated
somewhat, but very slowly
paddy, I'll try to do this and report results, seems that ketones are formed on calcium salts of organic acids, it would be interesting to form some
resin ketones 
|
|
|
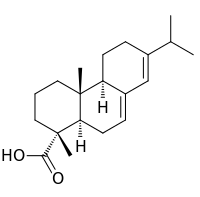
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have just found an interesting section of a book in the SM library, check out Fieser; Experiments in organic chemistry 1941 p265 for the isolation
of abietic acid from pine resin. Sounds quite interesting and easily do-able. It looks like the yield should be about 10% if all of the material from
one stage is used in the next. In PATR there is a mention of the nitration of abietic acid with fuming nitric acid to give a yellow poly-nitro
derivative that forms red explosive salts; interesting.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
This thread isn't in <strong><a href="forumdisplay.php?fid=3">Energetic Materials</a></strong>; your acronyms are meaningless.
What is PATR?
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I collected some chunks of pine resin the other day which had fallen from a damaged tree limb about 5 meters above the ground. Well over a meter of
the limb is heavily coated with sap for some reason. Some of the sap is friable while other pieces quite sticky. It has a pleasant scent not so
overpowering nor straight turpentine smell.
Some of these rosins are quite colorful.
https://en.wikipedia.org/wiki/File:Rosins.JPG
https://en.wikipedia.org/wiki/Rosin
It's interesting how this electret undergoes polarity reversal. Imagine how unusual/confusing this was, before this electrostatic characteristic was
expected.
http://www.youtube.com/watch?v=1DR-tTU8uIM#t=3m57s
 
|
|
|