| Pages:
1
..
32
33
34
35
36
..
40 |
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Something interesting with film interference:

I had made a reaction in an ionic liquid and after it I have extracted it with toluene. After a while the flask looked like this and between the two
phase there was a rainbow like film as seen on the picture. Anyone ever seen such a thing?
Thin film interference is common at the surface of metals (e.g.: bismuth) and with some organics on the surface of water as everyone knows, but I have
never heard and never seen it before between two massive layers.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kristofvagyok  | | Thin film interference is common at the surface of metals (e.g.: bismuth) and with some organics on the surface of water as everyone knows, but I have
never heard and never seen it before between two massive layers. |
I think you should be able to conclude that
there's a third, very thin layer between the two. You need two boundary changes to get interference.
It would be a good demonstration, though, to make something replicable and stable along these lines. You'd need three liquids, all mutually
immiscible, and yet able to wet each other. Just a fraction of a drop of the middle-density liquid would do it.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Are you sure what you're seeing isn't simply the meniscus of the bottom liquid? You claim to see a rainbow but I find it hard to see... One way to
resolve it is to pour the liquids into a narrow cylindrical container, that should definitely reveal any third phase.
[Edited on 26-4-2013 by blogfast25]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Thermochromic properties of bis(diethylammino) tetrachlorocuprate(II)
Green color at room temperature
Yellow color at >50*C(heated by a space-heater)
Taken from a melting point capillary with 20x zoom.
 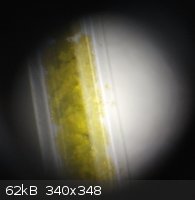
[Edited on 27-4-2013 by smaerd]
|
|
|
togipaw
Harmless

Posts: 5
Registered: 20-3-2013
Location: Alaska
Member Is Offline
Mood: No Mood
|
|
Bismuth Crystallization
Goofing around with Bismuth. Not the classic example of hopper crystals but I like how it turned out.

|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
1g etn before recrystallization:

[Edited on 4-28-2013 by chemcam]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
1g of what?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I was wondering too, but not daring enough to ask.
Lets see, 1g of puryfied white powder. If it was not on a chemistry forum...
[Edited on 28-4-2013 by plante1999]
[Edited on 29-4-2013 by plante1999]
I never asked for this.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Haha I left the name out to see if anyone thought cocaine. But its ETN. I have it recrystallized now but I'm having a hard time taking a close up shot
of the needle like structure. It blurs together and just looks like a clear mass of crystals.
1g etn after recrystallization:

[Edited on 4-29-2013 by chemcam]
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Here's a couple of pictures of some selenium dioxide I prepared recently. The sublimed oxide gives some nice (Toxic) needle like crystals. The SeO2
was prepared from elemental selenium using nitric acid.
 
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Very nice pictures, did you photograph on a mirror like I have been? You must have an excellent camera to take such detailed close-ups!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by smaerd  | Thermochromic properties of bis(diethylammino) tetrachlorocuprate(II)
Green color at room temperature
Yellow color at >50*C(heated by a space-heater)
Taken from a melting point capillary with 20x zoom. |
Very cool- how did you make it?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Synthesis of the Thermochromic Compound
1.35g(0.00791mol) of Copper(II) chloride dihydrate was weighed and added to a beaker containing 3.0mL of denatured ethanol. 2.18g(0.0198mol) of
diethylamine hydrochloride was weighed and added to 15.0mL isopropanol in a separate beaker. Both solutions were covered with a watch glass and gently
warmed on a hot-plate until the solids were no longer visible. While warm the ethanolic solution was poured into the diethylamine hydrochloride
solution. The beaker was rinsed with warm ethanol and the rinses were transferred to the solution.
The solution was allowed to cool to room-temperature then chilled in a ice bath. A flock of glistening particles were visible. Scratching and 20
minutes of resting in an ice-bath did not induce crystallization. The beaker was boiled on a hot-plate with stirring to remove about a fifth of it's
volume. On heating the solution turned from emerald green to an amber brown. The brown solution was cooled and it returned to a green color. Fifteen
minutes in an ice-bath with scratching did not induce crystallization. The solution was left to evaporate for five days.
An orange viscous oil remained without alcoholic odor. 3.0mL of isopropanol was added to the solution. No parafilm was available to move the solution
to the lab freezer, so the solution was again left to evaporate for two days.
To the oil 2mL of chilled isopropanol was added. The solution was chilled in an ice-bath. A seed crystal was added. Immediate crystallization
occurred yielding a slushy green solid. The solid was vacuum filtered and rinsed with chilled isopropanol. The solid was dried between two pieces of
filter paper supported by dry paper towels. 0.52g(0.0021mol, 26.92%) of product was obtained.
---------------------------
If I were to repeat the experiment I would use stoichiometric amounts of cupric chloride and diethylamine HCl which is what the publication's
recommend(note two moles diethylamine hcl for one mole of cupric chloride produces one mole of complex). The anhydrous cupric chloride is preferred
because there is evidence to suggest the compound irreversibly hydrolyzes after prolonged exposure to water. Most of the time there isn't this big a
problem with the crystallization it's likely that the excess diethylamine hcl(known to recrystallize from ethanol) inhibited this and/or the solvents
were not dry enough.
I would also not try to boil the solution down(If the crystallization failed). A slow evaporation seems to work fine for yielding a solid mass which
can be rinsed with isopropyl alcohol on a vacuum filter.
Also the complex can be crashed from the solution using diethyl ether rather then crystallizing(which I guess is still crystallization).
Also note, anhydrous diethylamine HCl can be prepared from the free amine and hydrogen chloride(the gas). The fluffy white solid forms almost
instantly on exposure to the gas. Interesting to watch. It can be recrystallized from ethanol if necessary.
[Edited on 29-4-2013 by smaerd]
[Edited on 29-4-2013 by smaerd]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by smaerd  | Synthesis of the Thermochromic Compound
1.35g(0.00791mol) of Copper(II) chloride dihydrate was weighed and added to a beaker containing 3.0mL of denatured ethanol. 2.18g(0.0198mol) of
diethylamine hydrochloride was weighed and added to 15.0mL isopropanol.... |
Thanks for that. I don't have any diethylamine (or the hydrochloride); I wonder if tetraethylammonium chloride would work similarly.
I think the correct name would be diethylammonium tetrachlorocuprate(II), since the diethylammonium is a counterion, not a diethylamino ligand.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
The author of this publication doesn't share that naming convention 
It's kind of an unconventional compound as the hydrogen bonding from the cation highly influences the inner sphere geometry(hence the thermochromism)
Thermochromism in copper(II) halide salts. 4. Bis(diethylammonium)tetrachlorocuprate(II), structure of the high-temperature phase and physical
characterization of its two phases
Darrell R. Bloomquist, Mark R. Pressprich, and Roger D. Willett
Journal of the American Chemical Society 1988 110 (22), 7391-7398
http://pubs.acs.org/doi/abs/10.1021/ja00230a020
[Edited on 29-4-2013 by smaerd]
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Capsaicinoid Extraction, Step One
I am getting ready to extract the capsaicinoids from a bunch of habanero peppers. The picture is of the chopped up peppers drying out (2nd day). I
will post new pics as I complete more of the extraction since it takes multiple days to complete each step.

If it comes out nicely I will make a thread in pre-publications compete with photos and step-by-step instructions.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Potassium Ferrocyanide?
This is a pretty picture but I have a question also. I ordered 125g potassium ferrocyanide and the bottle says that but I was expecting yellow
crystals. Do you think they accidentally bottled ferricyanide or is this correct color for lab grade?
<img src="http://i1314.photobucket.com/albums/t573/chemcamtv/Chemistry/IMG_20130503_201753_566_zps74977b80.jpg" width="800" />
(edit)
When I smash up the crystals I get bright lemon yellow.
[Edited on 5-4-2013 by chemcam]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image width]
[Edited on 7/7/13 by bfesser]
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Yea, that's ferricyanide right there.
|
|
|
binaryclock
Hazard to Others
  
Posts: 121
Registered: 9-4-2013
Location: Canada
Member Is Offline
Mood: Organic
|
|
No, those look like cherry pop candy from the 80s!
Current Project: Playing with my new Laboy advanced distillery kit!
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Almost certainly potassium ferricyanide. Bad luck.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Yes that's potassium ferricyanide or possibly nitroprusside, its about that colour too. But where did you buy it? I'd be very worried about buying
anything again from them as that's a pretty fundamental error. Imagine the mixed up compounds were both say white there's the potential for a serious
incident.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Quote: Originally posted by Boffis  | | Yes that's potassium ferricyanide or possibly nitroprusside, its about that colour too. But where did you buy it? I'd be very worried about buying
anything again from them as that's a pretty fundamental error. Imagine the mixed up compounds were both say white there's the potential for a serious
incident. |
This is a very large company, I have bought a lot of stuff from them and never had any problems but next time I go there I will definitely bring it
up. I would say if its wrong it the equivalent of a pharmacist dispensing the wrong pills.
I still am not certain what it is. When I smash the crystals up they go lemon bright yellow and stain white paper yellow. I am having trouble finding
info on the ferro and ferricyanides does anyone have a test I could perform to be absolutely sure or good literature? I don't have access to
references. I was going to make Prussian blue but I read both ferro and ferri can make it.
[Edited on 5-4-2013 by chemcam]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Use ferric chloride/sulphate; a blue coloured ppt indicate ferrocyanide (Turnbulls = Prussian blue). Ferricyanide don't ppt unless very concentrated
if I recall correctly.
With ferrous salts (if very pure) you get a white ppt but it rapidly turns blue through oxidation
Ferricyanide often react with redox indicators eg starch iodide, tolidine etc
Nitroprussides give an intense purple colour with alkali sulphides and a pinkish colour with alkali sulphites (not metabisulphites).
Nitroprussides also give coloured reaction with many organic chemical and in the past was widely used to test for some of them.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Maybe the outer surface oxidised to ferricyanide?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i thought chlorine made ferricyanide from ferrocyanide and also isnt ferricyanide more expensive?
|
|
|
| Pages:
1
..
32
33
34
35
36
..
40 |