| Pages:
1
..
3
4
5 |
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Has anybody ever tried oxidizing nitroso R salt with oxone with or without the presence of acetone? This seems to be the miracle oxidant for most
things.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
R-salt as reagent for nitrosation of hydrazine?
Today I have been wondering what may be the result of reacting R-salt with hydrazine. Nitrosamines can react with hydrazine to form the azide group
or free hydrazoic acid.
For example diphenylnitrosamine reacts with hydrazine in the presnce of sodium hydroxide to form sodium azide while the reacting nitrosamine precursor
is reduced to the amine. The scenario is one where the nitrosamine acts as a reagent for nitrosation or diazotization. I have speculated before that
R-salt may have usefulness as a reagent for nitrosation and it could possibly work for such purpose. See attached patent DE273667 for reference for
the diphenylnitrosamine reaction with hydrazine.
Attachment: DE273667 Staudinger Azide from nitrosamine and hydrazine patent.pdf (104kB)
This file has been downloaded 933 times
[Edited on 27-4-2013 by Rosco Bodine]
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Rosco
What an interesting idea, I have a try at this one when I get home. The result I guess would be 1 mole of triazine and 3 of sodium azide per mole of
CTMTNA. I'll translate the patent tonight if I get time it's only 2 pages, I'm working on the Curtis paper from the azide thread at present but
25pages is heavy going. The advantage of diphenylamine appears to be it ease of recycling, I'm not sure that this nitroso compound will be recyclable
though its easy enough to prepare from hexamine.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yeah that is how I see it, one mole of R-salt should do the same nitrosation as would require 3 moles of diphenylnitrosamine, for having 3 nitrosamine
groups on the R-salt compared to the 1 of diphenylnitrosamine.
It is intriguing what may result and I can find nothing instructional in the literature. It would seem on first glance that the R-salt would simply
be reduced to hexamine from which it came, and that would be the byproduct of using R-salt as a nitrosating agent for the hydrazine. I would amend
that first impression after reviewing the structure, and seeing that it will not occur that hexamine will be the byproduct because the original
hexamine structure was lost in the synthesis of the trimethylene structure of R-salt. The likely byproduct of nitrosation would seem to be probable
trimethylenetriamine. Not being sure what would be the hydrolytic stability of the R-salt, nor knowing what the kinetics would be, it could be a
"dump in a lump" scenario for the addition of the R-salt, or incremental addition where one would work better or equally well being an unknown.
Also there is the matter of the R-salt decomposition trimethylenetriamine byproduct being separated or decomposed or perhaps just left in solution, it
would depend upon what is intended later for isolation of the ultimate intended product. It just seemed like a possible good candidate for an aqueous
reaction system which could produce a decent yield of azide.
I have attached a fair amount of documents into the azide thread recently, and I am still reading and requesting yet other references and abstracts.
Thanks for any help with insight from the translations of especailly interesting parts. Azides are a bit of very interesting chemistry which overlaps
the inorganic and organic realm.
Analogously the R-salt may also serve to nitrosate hydrazodicarbonamide (biurea) which should have some solubility in alkaline solution.
Similarly R-salt may nitrosate acetone azine or methyl ethyl ketazine in aqueous alkaline solution.
Possible experiments here  I think 'em up don't I ? I think 'em up don't I ? 
[Edited on 27-4-2013 by Rosco Bodine]
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Yep, Iève been thinking about this too and I think you are right about the hydrolytic stability of the byproduct cyclo-trimethylenetriamine. It is
likely to break down into formaldehyde and ammonia both of which are volatile and would be removed with the alcohol (and then recombine to form
hexamine and excess ammonia) leaving behind relatively pure sodium azide. Mmmmmm sound too good to be true(!) but certainly worth trying. I wonder if
dimethylnitrosamine would work? then the freed amine would certainly be volatile with the alcohol.
Here's the translation of Staudinger's patent (btw where did the number DExxx come from? The pat number in the document is different.
[Edited on 27-4-2013 by Boffis]
Attachment: Hydrazoic acid & azide salts from hydrazine & nitrosamines H Staudinger 1913 Patent DE273667 English.docx (15kB)
This file has been downloaded 923 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
DE205683 is the Stolle azide patent that DerAlte and I have already translated, so you got your wires crossed there. The Staudinger patent above is
the nitrosamine plus hydrazine related patent DE273667 also same on the patent.
Staudinger is simply referencing the earlier Stolle patent as prior art. So there's your confusion. Just edit the main number to DE273667.
Thanks Boffis for translation of the patent. I changed the formatting slightly to fit it on a single page and made a pdf for it too. (attached)
Urbanski 3 has a writeup on R-salt that provides useful information about the hydrolytic properties (attached)
The Bachmann and Deno JACS article also mentioned earlier in the thread is attached
Here also is an R-salt synthesis which I am not certain but I don't recall being posted before. From Journal of the Chemical Society 1889

Attachment: German Patent 273667 A method for producing hydrazoic acid or azide salts.pdf (45kB)
This file has been downloaded 978 times
Attachment: R-salt preparation JCS.pdf (164kB)
This file has been downloaded 1053 times
Attachment: Nitrosamine pages from Urbanski 3.pdf (273kB)
This file has been downloaded 4340 times
Attachment: Bachmann and Deno JACS nitrosation of hexamine.pdf (395kB)
This file has been downloaded 1180 times
[Edited on 27-4-2013 by Rosco Bodine]
|
|
|
Energetic Einstein
Harmless

Posts: 12
Registered: 10-1-2014
Member Is Offline
Mood: No Mood
|
|
Does anyone happen to know the approximate "self-life" of stabilized R-salt? If not can anyone lead me in the right direction to calculating it?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
What exactly is "stabilized" R-salt ? Please be more specific.
|
|
|
packetforger
Harmless

Posts: 48
Registered: 21-2-2014
Member Is Offline
Mood: Condensing
|
|
IIRC, R-Salt can have a stabilizing effect on nitric esters (nitrated polyols).
Would like to confirm that, will come back with sources once I find them, if no one beats me to the punch.
EDIT: Relavent quote found, as suspected, Rosco himself!
Quote: Originally posted by Rosco Bodine  | From the information in the Bachmann & Deno article it appears that use of acetic acid takes the pH too far in the wrong direction for R-salt and
leads instead to the DPT material which is primarily useful as a precursor for HMX . The DPT is obtained in better yield more cheaply by simply using
less HCl to raise the pH of the
reaction mixture , so acetic acid offers no advantage .
PATR states that a mixture of R-salt and NG kept at 90C for 5 days showed no apparent decomposition ? Possibly a misprint , because that would seem
to indicate stabilizing properties for R-salt towards nitroesters which if true is very interesting . If this is general , R-salt could possibly have
value as an energetic stabilizer as well as filler for ETN melt and other low melting compositions , and possible usefulness in many other nitroester
containing compositions . |
[Edited on 6-4-2014 by packetforger]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That information was from PATR and identified as a possible error / misprint. I never tested the reported R-salt use as a stabilizer or looked into
further. That reference was something that was referencing a possible anomaly where the R-salt would be acting as the stabilizer, or there could be a
reciprocal stabilizing effect, but I never found anything further about that or determined by any experiments if it is even true or is a misprint. I
remain skeptical and would guess it is probably a misprint.
[Edited on 6-4-2014 by Rosco Bodine]
Attachment: R-salt pages from PATR Vol. 3 C (cont.)-D.pdf (389kB)
This file has been downloaded 1441 times
|
|
|
Energetic Einstein
Harmless

Posts: 12
Registered: 10-1-2014
Member Is Offline
Mood: No Mood
|
|
Sorry about that. I'm curious on what the general "Shelf-life" of r-salt is.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
3-5 years under mild storage conditions
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Washed only material turned to something like hexamine after 1 year of room temperature storage and some time at elevated temperatures. Also, I've
heard it's difficult to set off generally, so it's not a good cap basecharge option.
|
|
|
Keith Fletcher
Harmless

Posts: 29
Registered: 3-10-2014
Location: Eastern US
Member Is Offline
Mood: Brittle
|
|
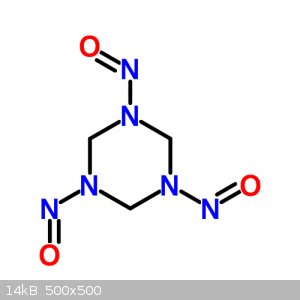
1,3,5-Trinitroso-1,3,5-triazinane (R-Salt)
1,3,5-Trinitroso-1,3,5-triazinane
Molecular Formula C3H6N6O3
Average mass 174.118 Da
Monoisotopic mass 174.050140 Da
ChemSpider ID 24566
Log Octanol-Water Partition Coef (SRC):
Log Kow (KOWWIN v1.67 estimate) = -1.78
Boiling Pt, Melting Pt, Vapor Pressure Estimations (MPBPWIN v1.42):
Boiling Pt (deg C): 407.93 (Adapted Stein & Brown method)
Melting Pt (deg C): 145.55 (Mean or Weighted MP)
VP(mm Hg,25 deg C): 7.75E-007 (Modified Grain method)
Subcooled liquid VP: 1.29E-005 mm Hg (25 deg C, Mod-Grain method)
Water Solubility Estimate from Log Kow (WSKOW v1.41):
Water Solubility at 25 deg C (mg/L): 1e+006
log Kow used: -1.78 (estimated)
no-melting pt equation used
Water Sol Estimate from Fragments:
Wat Sol (v1.01 est) = 1e+006 mg/L
ECOSAR Class Program (ECOSAR v0.99h):
Class(es) found:
Aliphatic Amines
Henrys Law Constant (25 deg C) [HENRYWIN v3.10]:
Bond Method : 1.69E-008 atm-m3/mole
Group Method: Incomplete
Henrys LC [VP/WSol estimate using EPI values]: 1.776E-013 atm-m3/mole
Log Octanol-Air Partition Coefficient (25 deg C) [KOAWIN v1.10]:
Log Kow used: -1.78 (KowWin est)
Log Kaw used: -6.161 (HenryWin est)
Log Koa (KOAWIN v1.10 estimate): 4.381
Log Koa (experimental database): None
Probability of Rapid Biodegradation (BIOWIN v4.10):
Biowin1 (Linear Model) : -0.9088
Biowin2 (Non-Linear Model) : 0.0001
Expert Survey Biodegradation Results:
Biowin3 (Ultimate Survey Model): 1.6590 (recalcitrant)
Biowin4 (Primary Survey Model) : 3.6520 (days-weeks )
MITI Biodegradation Probability:
Biowin5 (MITI Linear Model) : -0.3603
Biowin6 (MITI Non-Linear Model): 0.0000
Anaerobic Biodegradation Probability:
Biowin7 (Anaerobic Linear Model): 0.4760
Ready Biodegradability Prediction: NO
Hydrocarbon Biodegradation (BioHCwin v1.01):
Structure incompatible with current estimation method!
Sorption to aerosols (25 Dec C)[AEROWIN v1.00]:
Vapor pressure (liquid/subcooled): 0.00172 Pa (1.29E-005 mm Hg)
Log Koa (Koawin est ): 4.381
Kp (particle/gas partition coef. (m3/ug)):
Mackay model : 0.00174
Octanol/air (Koa) model: 5.9E-009
Fraction sorbed to airborne particulates (phi):
Junge-Pankow model : 0.0593
Mackay model : 0.122
Octanol/air (Koa) model: 4.72E-007
Atmospheric Oxidation (25 deg C) [AopWin v1.92]:
Hydroxyl Radicals Reaction:
OVERALL OH Rate Constant = 242.3450 E-12 cm3/molecule-sec
Half-Life = 0.044 Days (12-hr day; 1.5E6 OH/cm3)
Half-Life = 0.530 Hrs
Ozone Reaction:
No Ozone Reaction Estimation
Fraction sorbed to airborne particulates (phi): 0.0909 (Junge,Mackay)
Note: the sorbed fraction may be resistant to atmospheric oxidation
Soil Adsorption Coefficient (PCKOCWIN v1.66):
Koc : 911.8
Log Koc: 2.960
Aqueous Base/Acid-Catalyzed Hydrolysis (25 deg C) [HYDROWIN v1.67]:
Rate constants can NOT be estimated for this structure!
Bioaccumulation Estimates from Log Kow (BCFWIN v2.17):
Log BCF from regression-based method = 0.500 (BCF = 3.162)
log Kow used: -1.78 (estimated)
Volatilization from Water:
Henry LC: 1.69E-008 atm-m3/mole (estimated by Bond SAR Method)
Half-Life from Model River: 4.572E+004 hours (1905 days)
Half-Life from Model Lake : 4.988E+005 hours (2.078E+004 days)
Removal In Wastewater Treatment:
Total removal: 1.85 percent
Total biodegradation: 0.09 percent
Total sludge adsorption: 1.75 percent
Total to Air: 0.00 percent
(using 10000 hr Bio P,A,S)
Level III Fugacity Model:
Mass Amount Half-Life Emissions
(percent) (hr) (kg/hr)
Air 0.044 1.06 1000
Water 58.9 4.32e+003 1000
Soil 41 8.64e+003 1000
Sediment 0.116 3.89e+004 0
Persistence Time: 992 hr
My rule of life prescribed as an absolutely sacred rite smoking cigars and also the drinking of alcohol before, after and if need be during all meals
and in the intervals between them.
- Winston Churchill
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Regarding the stability of this compound, I have just come across the jar of recrystallised material figured in my post above and the beautiful yellow
crystals are in apparently perfect condition with no signs of degradation. So it would appear that properly purified and stored in a cool dry place in
a tightly closed jar it is certainly stable for at least 8 years.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Has anyone had success oxidizing TMTN to RDX?
I have tried:
Acetic Acid/Sodium Perborate
Fail-
No reaction
Acetic Acid/Ammonium Nitrate
Success?
foam volcano @70C after about 45m-1Hr, Solution changes color from lemon to white, no yield on crashing with water.
H2SO4/AN
Fail-
Instant decomposition
Acetic Acid/H20/H2SO4/AN
Success-
Solution foams turns color from lemon to white temp rises to ~70C and releases white fumes RDX (what I assume) appears to come out of solution even in
acid but on crashing with water, very little product.
Literature claims AN/H2SO4. I have trouble believing that. Has anyone successfully converted TMTN to RDX? Also, while Im in this thread, has anyone
made RDX from DPT? DNPT is much higher yielding from nitrosation of hexamine in acetic acid than is TMTN. I'm curious if a high yield RDX or HMX could
come from DNPT>oxidize to DPT>Nitration via WFNA to RDX/HMX. Of course that would hinge on first finding out how to properly oxidize TMTN or
DNPT, which is proving difficult.
[Edited on 9-11-2022 by Hey Buddy]
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 175
Registered: 12-7-2018
Member Is Offline
|
|
I think you should use nitric acid alone to oxidize it.
[Edited on 10-11-2022 by Alkoholvergiftung]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Nitric Acid does oxidize r-salt to rdx, according to Lawrence Livermore analytical RDX procedure with H2O2. However, nitric acid can simply form RDX
instead of R-Salt route. I have read many assumptions that R salt can simply "be oxidized" to RDX, but I get the impression some of this may be
speculation. I would enjoy synthesizing RDX in lower yield via TMTN foregoing the reliance of nitric acid.
There are a couple more routes, but they are getting whacky now that the conventional oxidization methods failed.
1) Electrolytic oxidation in ammonium sulfate bath (works very well with NQ reduction to amino guanidine with simple lead anode.)
2) Bubbling ozone through r salt solution
3) oxone (would like to avoid)
4) peroxy-nitrite from NaNO2, HCl, perborate or percarbonate
Literature also claims AN/H2SO4 will oxidize R Salt to RDX but I have not had success. I am curious if *ANYONE from SM has had success with ANY
method?
[Edited on 10-11-2022 by Hey Buddy]
|
|
|
| Pages:
1
..
3
4
5 |