| Pages:
1
2
3
4 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Anyone tried an 'IR intensive' but VIS transparent aromatic compound yet?
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Quote: Originally posted by Endimion17  | Quote: Originally posted by Eddygp  | I would like to see something which is transparent in visible light but opaque in IR... or UV (glass)
[Edited on 13-4-2013 by Eddygp] |
You have to specify which IR and which UV. They differ pretty much.
NIR, MIR, FIR, UVA, UVB, UVC, it's a wide whoice.
Borosilicate glassware is NIR-transparent but FIR-opaque (maybe even for MIR, I'm not sure). It's also semitransparent to UVA, but opaque to UVB and
UVC.
NIR is "security cam IR" and the rest is in the domain of thermal imaging, so to say. |
I meant NIR and UVB.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
I'm uploading a new video. It will be ready in ten minutes or so.
<iframe sandbox width="420" height="315" src="http://www.youtube.com/embed/gvdMe4vtsIY" frameborder="0" allowfullscreen></iframe>
I just hope YouTube won't delete it because of the soundtrack...
Has anyone tried viewing NIR absorptive liquids using special equipment yet?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
As promised.
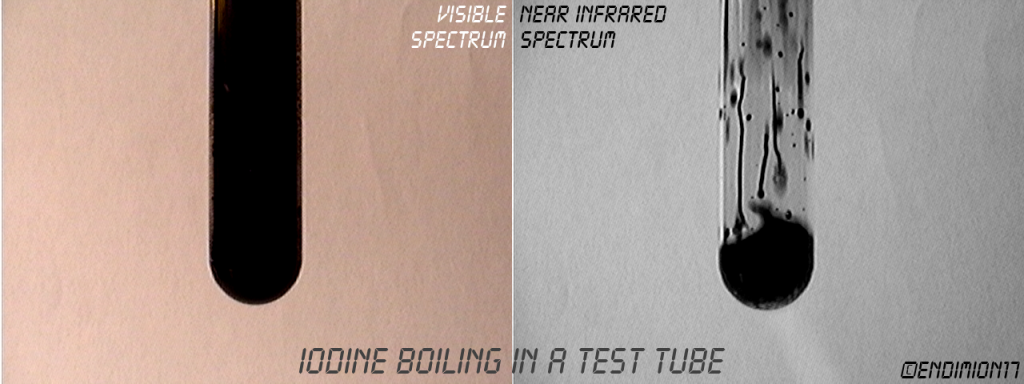
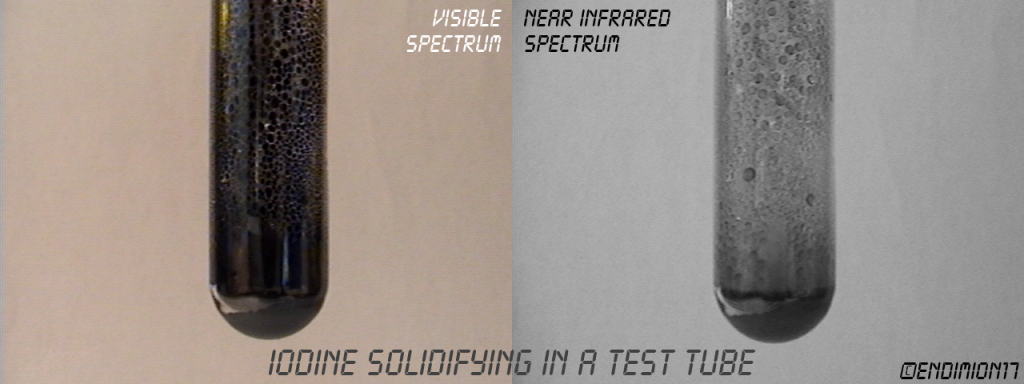
Iodine vapor is transparent, and liquid iodine is opaque. Solid iodine transparency depends on the degree of order in the crystals.
It's fun to watch it boiling on the screen when all you can see in the tube is blackness.
Video coming soon.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'm no expert on NIR spectroscopy but part of the of the I2 NIR absorbance must come from I2 as a quantum rotational (rotor) system, I think. Oh and
these quantum harmonic oscillators too 
I've put the video on my blog.
[Edited on 17-4-2013 by blogfast25]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Eddygp  | I would like to see something which is transparent in visible light but opaque in IR... or UV (glass)
[Edited on 13-4-2013 by Eddygp] |
Get a mirror.
To a bad, but interesting, approximation, the cornea of your eye meets those criteria.
Indeed, it's one of the factors that defines the ends of the visible spectrum (more so at the UV end, than the IR.)
the retina is sensitive to UV, but little reaches it because of absorbtion in transit through the eye: the proteins absorb it.
The IR is attenuated by water present in the eye and also by the proteins.
If you look at the spectum about two thirds of the way down this page
http://www.lsbu.ac.uk/water/vibrat.html
you can see that "visible" light is a pretty good match to the gap in water's absorbtion.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I've been told about a demo involving a UV lamp, a sheet of fluorescent TLC plate stuff, and a beaker of hot toluene. Mount the TLC plate on the
wall, put the beaker under it, light it up with the UV lamp, and the invisible vapours from the toluene cast a visible shadow on the plate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Forget what I said about the benzene and other aromatics- these absorb in the UV, not in the IR range of the spectrum!
I remembered this incorrectly. Benzene would most likely be as transparent in IR as in visible light.
Benzene vapor could be made visible by the same technique as for mercury vapor: between a UV light source and a fluorescent screen.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by garage chemist  | Forget what I said about the benzene and other aromatics- these absorb in the UV, not in the IR range of the spectrum!
I remembered this incorrectly. Benzene would most likely be as transparent in IR as in visible light.
Benzene vapor could be made visible by the same technique as for mercury vapor: between a UV light source and a fluorescent screen.
|
Huh?
Benzene and aromatics do have strong absorbances in IR. Not sure what you mean here...
|
|
|
| Pages:
1
2
3
4 |