| Pages:
1
..
3
4
5
6 |
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
translate
first paragraph
Akahori, etc., by heating in pyridine (2) benzaldehyde N-methyl alanine (1) in 1942,
While low yield (16%) reported that (3) is obtained ψ-ephedrine (Scheme 1). Takagi, etc. Then one Emoto
This reaction has been studied in detail thus. But 2, 3, ephedrine is obtained in 29% yield as a mixture of isomers
In asked, it remained still low yield. 3 This reaction with carbon on the asymmetric carbon in the decarboxylation of amino acids -
Form a unique reaction produces carbon bond not only a [A], as the basic skeleton of the drug contains many important
Since the amino alcohol can be obtained by one stroke from unprotected amino acids, and is considered a very interesting reaction
The example.
second paragraph...
Where noted in this reaction was examined again, we see that Okizazorijin (5) is produced as an intermediate
The out without isolating this 5, hydrolyzed with 5% AcOH, amino alcohol diastereomers body (6)
Was able to get a good yield as a mixture of diastereomers (Table 1). In other words, 1 and 6.5-fold molar Benzuaru
After heating to 130oC in the absence of a solvent dehydrogenase (4), 5% AcOH aqueous solution was added to the reaction solution directly without
intermediate extract
Was heated, the desired product was obtained in a yield of up to 84%. We have reported these results in this debate last year. Time 4
I want to report that examined in detail the reaction conditions for this reaction further.
third paragraph
Using (7) piperonal good yield most of the benzaldehyde derivative, algae solvent, reaction temperature, and 7
The reaction was carried out by changing the number Le, the reaction time (Table 2). The reaction proceeds at all EtOH water as a solvent and a protic
The reaction proceeds in a solvent CPME (Run 1, 2), the ether is a polar solvent DMF, DMSO or was not
I (Run 3-9). 78% in DMSO solvent was obtained in good yield and most (Run 4). Also, if DMSO, the DMF
(Run 3, 4), in, the anti-aldehyde yield almost the same in two equivalent reaction proceeds even if the reaction temperature is lowered to 85 oC
It was found that the response is in progress (Run 7, 9). In any reaction conditions, there was no of diastereoselectivity. In the absence of a
solvent
4th paragraph
Since obtained in 87% (1, Run 6 Table) is performed when the reaction was seen some decrease in the yield, obtained this
The result was, on finding a condition for allowing the reaction to proceed stereoselectively or diastereoselective is very
It is considered to be a useful finding.
In addition, N-methyl phenylalanine (9), N-
Rui alanine methyl-leucine (10) such as (11)
Reaction was also investigated, and the NMR, the target compound
That have been obtained, but is coming in at the check, generated
Is difficult, a place now are considering separation method
Is filtered.
wonderful paper... shows the carbanion in first picture
and intermediate in another
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Translating this paper from Japanese is difficult ,even with Google translator,But it seems using polar solvents Like DMSO or DMF increase Yield if
Piperonal used(benzaldehyde didn't mention)
Also It seems 5%AcOH aqueous solution hydrolyze intermadiate and increase Yield(@Quantum_Dom used 25%AcOH in Toulene.I dont know which one has better
yield)
I want to try this method but i am not sure to use DMSO as solvent.somebody has idea?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hilski  | Holy shit!
This would lead one to believe that yields in the range of 60%-70% are possible, given the right conditions. |
Those yields are based on the limiting reagent (obviously!), which in this case is N-methylalanine. So their preparative value can be insignificant
for the reaction with substituted benzaldehydes.
Besides, this article and these same issues were already discussed at this forum in some other thread.
The yield using N-methylalanine and 6.5 eq. benzaldehyde under classical conditions (neat, 130 °C) gave them a 48% yield of the 1:1 diastereomeric
mixture of products. This is comparable to the 35% obtained by QuantumDot on the related reaction of 6 eq. of benzaldehyde with alanine.
Furthermore, the tables in the article indicate that no solvent compares in efficiency to the use of neat reaction conditions. For example, on
piperonal, the use of DMSO gave the best yield of 78%, while the classical conditions with the same excess of the aldehyde gave them 87% yields. The
only good property of DMSO that is reported there, is that the reaction runs best already at 80 °C and that at the stoichiometric ratio of reactants
it still gives a good yield. But the article does not report yields for the same 1:2 ratio under neat conditions, so even this is dubious. After all,
the same ratio/yield influence was also found for the trivial non-polar solvent cyclopentyl methyl ether.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
The yield using N-methylalanine and 6.5 eq. benzaldehyde under classical conditions (neat, 130 °C) gave them a 48% yield of the 1:1 diastereomeric
mixture of products. This is comparable to the 35% obtained by QuantumDot on the related reaction of 6 eq. of benzaldehyde with alanine.
Furthermore, the tables in the article indicate that no solvent compares in efficiency to the use of neat reaction conditions. For example, on
piperonal, the use of DMSO gave the best yield of 78%, while the classical conditions with the same excess of the aldehyde gave them 87%
yields. |
Thanks.
I kinda figured that out after I made that ignorant post earlier.
I suppose QuantumDot's experiments are the most successful example of this type of procedure that I have seen, (other than in the literature) even at
35% yields.
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I dont want to start new topic and i prefer to ask my question here.
somebody tried tetrahedron letter on N-methylation L-alanine by formaldehyde + Zinc?
I Like to test this method but i dont have detail for this reaction,this letter has little info about reaction detail
Attachment: Zn_HCHO_methylation.pdf (98kB)
This file has been downloaded 1768 times
[Edited on 24-9-2012 by Waffles SS]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by Quantum_Dom  | This thread is dedicated to those who do desktop research and solely base theyre argument on theory. It is also dedicated to my good friend and
colleague Xtaldoc.
This subject is a favorite of mine. It’s a very nice (and hard) problem to tackle. Such an elegant and OTC mean of obtaining racemic
phenylpropanolamine (PPA) and yet so many obstacles and problems regarding proper work-up, understanding of the overall mechanism or the nature of the
byproduct formation. Not to mention a less than respectable reported molar yield of 15 % in average! I spent a lot of time studying and trying to
tweak the reaction protocol and finally I am very happy to report a 35 % molar yield of racemic PPA*HCl (based on alanine). I apologize to the
moderators for opening a new thread in advance but I figured the previous one was rather old and convoluted. Feel free to merge it with the old one if
its not worthy of a new thread.
This has been discussed over and over at many different sites and I am sure you are well aware of it. This is why I will solely base my post on the
Cycloknight's thread [1] as I believe it is the most complete and up to date on the subject. Furthermore, my improved work-up and yield is directly
inspired and based on CycloKnight’s write-up, the Yokoyama et al. article [2] and some comments that Nicodem made in the Cycloknight's thread [3].
Before I get to the actual protocol I need to make a few remarks which will make (I hope) the various steps in my procedure more transparent and
justifiable.
1-Lots of people are arguing that the only compound obtained in this procedure is 1,2-diphenylethanolamine (DPEA) quoting this study and affirming that no PPA is obtained at all. DPEA is indeed the major product but it is easily removed in the work-up. The reason the
authors are not isolating any PPA is mainly due to 1) the very little scale theyre working on (amount of PPA is almost insignificant at this scale),
2) they are omitting to concentrate the aqueous phase extracts in the work-up (the latter contains PPA*HCl). This will be re-explained later and will
prove by the same occasion that referring to that article, to conclude that PPA is not in fact produced, is incorrect.
2-The Yokoyama article mentions that the stoechiometry of the reaction involves two moles of benzaldehyde; one for the formation of PPA and one that
condense with the latter to form an oxazolidine. The latter can further be hydrolyzed in the work-up, to regenerate PPA and benzaldehyde, by refluxing
the whole mixture, after the condensation/decarboxylation is over. A large excess of benzaldehyde (6 equivalents) is also needed to maximize yields.
3-An important point was made by Nicodem in the CycloKnight’s thread regarding the possibility of an acid-base reaction between alanine and PPA. PPA
being a base (primary amine) and alanine having a carboxylic acid moiety, it is not impossible for the two to combine and form a salt that will surely precipitate in benzaldehyde. This topic was discussed and illustrated by me and Naf1 at The Vespiary
[4]. Knowing that in CycloKnights work-up all solids after the decarboxylation are assumed to be unreacted alalnine, and therefore discarded, it is
normal to wonder if theyre is not actual PPA*alaninate thrown away at the same time.
It is basically on those last two points that I believe my work-up of the reaction is superior, cleaner and brings a better yield of clean racemic
PPA*HCl. Heres the protocol:
In a 1 L RBF, 358 g of benzaldehyde (3.36 moles) and 50 g of finely crushed DL-alanine (0.56 moles) were mixed together with magnetic stirring. It was
attached to a simple distillation setup and heated on an oil bath at 140 celsius for 3 hours [5] (Figure-1). No more CO2 was evolved from the flask at
this point and the color of the mixture was a deep burgundy (almost black). The flask was removed from the oil bath and let cool on its own for 20
minutes. Afterwards, 100 mL of 20 % AcOH in Toluene were added to the flask and the latter was stirred, at RT, for 20 minutes [6]. When this induction
period was over, 400 mL of 15 % HCl (aq) was added to the flask and the whole mixture was refluxed gently on the oil bath for 3 hours. Afterwards, the
pH of the mixture was verified to be strongly acidic. If not, small portions of concentrated HCl (aq) were added until it was and the mixture was
refluxed for another 30 minutes. The flask was then removed from the oil bath, the mixture left to cool for a bit and then rapidly separated with a
separation funnel while still warm [7] (Figure-2). An orange/red aqueous phase and a dark black organic phase were obtained [8] (Figure-3).
The aqueous phase was washed 3 times with 150 mL of DCM [9] (Figure-4). Then the volume of the aqueous phase was reduced, with gentle heating and
stirring, until half of the volume was vaporized [10] (Figure-5). The remaining aqueous phase was washed 3 other times with 75 mL DCM. The aqueous
phase was then treated with small portions of NaHCO3 [11] until fizzing becomes less violent (Figure-6), then small portions of NaOH were added until
pH was strongly alkaline. A light brown-yellow oily layer with a strong and biting amine smell separated from the aqueous phase (Figure-7). The latter
was separated and the aqueous phase was extracted 4 times with 200 mL DCM [12]. All the NP fractions were pooled together, washed 2 times with 100 mL
BRINE and the DCM was stripped off to afford a viscous brown oil that partially turns to a solid once cooled [13].
The oil was dissolved in 5 times its volume of dry acetone. Small portions of an 1:3 HCl (muriatic)-isopropanol solution were added until pH 4 was
reached [14]. Large amounts of crystalline material precipitated during the addition (Figure-8 and 9). The flask was put in the freezer overnight, the
crystalline material was vacuum filtered and washed with small portions of cold and dry acetone. 33.2 g of racemic PPA*HCl were obtained (Figure-10).
Evaporation of the acetone liquor afforded another 3 g after appropriate washing and drying. Melting point, after 1 week in the dessicator, was
131-136 0C [15]. The product gave a positive test to Chen’s reagent and to the nitrous acid test [16]. It has a very distinctive smell reminding of
crude benzoic acid [17].
[1] http://www.sciencemadness.org/talk/viewthread.php?tid=5979
[2] https://www.thevespiary.org/talk/index.php?action=dlattach;t...
[3] http://www.sciencemadness.org/talk/viewthread.php?tid=5979&a...
[4] https://www.thevespiary.org/talk/index.php/topic,714.0.html
[5] No changes were made to CycloKnights protocol except longer time for decarboxylation and higher amount of benzaldehyde used.
[6] Glacial acetic acid being soluble in toluene, this step was performed in order to protonate the oxazolidine dissolved in the excess benzaldehyde
and make it more prone to hydrolysis latter on. I have no evidence if this step is necessary but I was inspired by the Yokohama et al. article were it
is used.
[7] The two phases separate very cleanly while still warm ( 50-55 0C). If the phases are left to cool at RT, the whole mixture turns into a nasty
emulsion that wont separate and heating needs to be reapply to separate the phases.
[8] The aqueous phase contains PPA salts, DPEA salts and other water soluble impurities. The NP layer contains a large amount of tar and lots of
unreacted benzaldehyde which can be vacuum-distilled later on by firstly washing a few times with diluted aqueous sodium carbonate the NP layer.
[9] This steps is really important as it removes most of the DPEA salts, chloroform is also equally good.
[10] This step is crucial as PPA freebase is soluble in water and will therefore allow one to maximize extraction efficiency once the freebase will be
released. An Erlenmeyer or a large flask with a neck is recommended here to minimize aerosol lost. Do not bring the solution to a boil either. The
temperature was 80-85 0C and it took roughly 5 hours for evaporation. Evaporation under vacuum would be of course ideal.
[11] The resulting aqueous solution being strongly acidic, the use of sodium bicarbonate allows for smoother neutralization of the excess acid (less
heat is generated). Also, a greater amount of salt will be present in the solution and will force the PPA freebase to separate more efficiently.
[12] Do not dry the DCM fraction with magnesium sulfate as a gelatinous addition complex is obtained.
[13] This is mostly due to the fact that the oil is a mixture of 4 different isomers with different melting points.
http://www.thevespiary.org/rhodium/Rhodium/chemistry/nor-pse...
[14] Gassing is not recommended here to claim the hydrochloride.
http://www.thevespiary.org/rhodium/Rhodium/chemistry/nor-pse...
[15] Reported melting point: 134-137°C (butanol-ether (50:50 v/v))
http://www.thevespiary.org/rhodium/Rhodium/chemistry/nor-pse...
[16] Chen’s reagent is specific for alpha-hydroxy amines such as (pseudo)ephedrine and PPA’s. Nitrous acid test indicates a primary amine.
[17] PHENYLPROPANOLAMINE HYDROCHLORIDE, ANALYTICAL PROFILES OF DRUG SUBSTANCES VOLUME 12, Isadore Kanfer, John M. Haigh, and Roslind Dowse
Please post any comments, critique and/or interrogations. I would love to discuss it with anyone interested in
optimizing yields furthermore or reproducing my results.- QD
|
I tried this method and i tested result by GC-MS. i attach result picture.
Someone can find out what is result?
Attachment: result.zip (44kB)
This file has been downloaded 998 times
[Edited on 14-10-2012 by Waffles SS]
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
The second spike on the attached JPEG (Designated (B) on the image) is supposed to be phenylpropanolamine. Not sure if this helps you or not.

Fig. 4. Ion chromatogram of l-TPC-derivatives: (+)-Cathinone (A), (+)-Phenylpropanolamine (B), (−)-Cathinone (C), (−)-Phenylpropanolamine
(D), (+)-cathine (E), (+)-Ephedrine (F), (+)-Ephedrine-d3 (G), (−)-Ephedrine (H), (−)-Pseudoephedrine (I), (+)-Pseudoephedrine (J) (all as
l-TPC-derivatives).
The attached JPEG is from:
Enantiomeric determination of ephedrines and norephedrines by chiral derivatization gas chromatography–mass spectrometry approaches
Journal of Chromatography B
Volume 825, Issue 1, 15 October 2005, Pages 88–95
http://www.sciencedirect.com/science/article/pii/S1570023205...
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Tell your analyst he is too incompetent for his job.
That is not a GC-MS! It is just a useless picture of a chromatogram with no relevant data and without peak assignations. And where are the
chromatograms of standards?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by Nicodem  |
Tell your analyst he is too incompetent for his job.
That is not a GC-MS! It is just a useless picture of a chromatogram with no relevant data and without peak assignations. And where are the
chromatograms of standards? |
Sure this is GC-Ms result(My friend worked with it and i saw this procedure)
We bought Hewlett Packard 6890/5973 one week ago and my friend is working with it.
also this is another result paper(it contain different peak and different chemicals name by this peak )
Attachment: result.zip (103kB)
This file has been downloaded 804 times
[Edited on 16-10-2012 by Waffles SS]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  | Sure this is GC-Ms result(My friend worked with it and i saw this procedure)
We bought Hewlett Packard 6890/5973 one week ago and my friend is working with it.
also this is another result paper(it contain different peak and different chemicals name by this peak ) |
You still do not understand what I talk about. GC-MS stands for gas chromatography–mass spectrometry. What you showed is only a chromatogram
useless by itself, and no mass spectroscopic data.
Your latest attachment is just the automatic list of candidates from the database. Again, what you don't understand is that if you want others to
interpret your data, you need to offer all the relevant data, not just pieces of puzzles that isolated have little meaning.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
These are MS Spectrums files
I think GC-Ms cant detect PPA.Hcl
(because this is ionic compound and i should use HPLC-LCMS)
Attachment: MS Spectrums.zip (898kB)
This file has been downloaded 828 times
[Edited on 17-10-2012 by Waffles SS]
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  | These are MS Spectrums files
I think GC-Ms cant detect PPA.Hcl
(because this is ionic compound and i should use HPLC-LCMS)
[Edited on 17-10-2012 by Waffles SS] |
Ummmm....Yes, PPA.HCL (and every other phenethylamine salt) can be detected by GC-Ms. GC-Ms is the main method used by labs to confirm positive
results from drug urinalysis screenings.
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hilski  | | Ummmm....Yes, PPA.HCL (and every other phenethylamine salt) can be detected by GC-Ms. GC-Ms is the main method used by labs to confirm positive
results from drug urinalysis screenings. |
That's not true. Amine hydrochlorides are not injected into GC-MS without prior derivatization into the free amine base or other volatile derivatives.
You generally do not want to detect pyrolysates, but the original analytes instead. Even so, amines sometimes give poor quality peaks and are not
always suitable for GC, even if they are volatile like 2-hydroxy-1-phenyl-propylamines. That's why they are sometimes derivatized to non-amine
analytes first, though in my experience, most of them give completely decent GC peaks as amines already. If Waffles SS works with such an incompetent
analyst that he does not even know what kind of analytes are suitable for GC-MS, then the best thing to do is change the analyst. Better yet, change
the chemist as well, because a chemist would give the standards to the analyst (like the starting materials and expected amine products: benzylamine,
2-hydroxy-1-phenyl-propylamines and their corresponding phenyloxazolidines).
result of 5 seconds of googling:
| Quote: | Gas chromatography has been extensively used as a method for determining phenylpropanolamine in pharmaceutical preparations and, to a lesser extent,
for the determination of the drug in body fluids. The drug has been chromatagraphed directly without derivatization and also as the silyl,
pentafluorophenyloxazalidine, acetone, butanone, trifluoroacetyltrimethylsilyl, heptafluorobutyryl and 2,6-dinitro-4-trifluoromethylbenzenesulphonic
acid derivatives. The gas chromatographic conditions are listed in Table 2.
[references are in the table]
cited from I. Kanfer, J. M. Haigh, R. Dowse, Analytical profiles of drug substances, vol. 12, 357 |
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | That's not true. Amine hydrochlorides are not injected into GC-MS without prior derivatization into the free amine base or other volatile derivatives.
|
I stand corrected. I didn't realize that samples of the the specific compounds in question were normally derivatized. But, as you stated, "most of
them give completely decent GC peaks as amines already" so I wouldn't think it necessary in this case, but maybe I'm wrong. Probably.
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
| Quote: |
1-Lots of people are arguing that the only compound obtained in this procedure is 1,2-diphenylethanolamine (DPEA) quoting this study and affirming
that no PPA is obtained at all. DPEA is indeed the major product but it is easily removed in the work-up. The reason the authors are not isolating any
PPA is mainly due to 1) the very little scale theyre working on (amount of PPA is almost insignificant at this scale), 2) they are omitting to
concentrate the aqueous phase extracts in the work-up (the latter contains PPA*HCl). This will be re-explained later and will prove by the same
occasion that referring to that article, to conclude that PPA is not in fact produced, is incorrect.
|
I tries this method with n-methyl alanine and i think this procedure is not perfect.
1-as Yokoyama mentioned ,I found out there is no need for refluxing solution with 15% HCl for 3 hours and just refluxing solution with 5% ACOH
/Toluene for 3-6 hours is sufficient(After that adding little 15% HCl can make hydrochloride salt of amines for separation step)
2- precipitation step should be done by HCl gassing(i got better result)
I dont know if we use N-methyl-alanine then DPEA is major product again or not? because when i used 1 mole(103gram) N-methyl-alanine and 6.5 mol
Benzaldehyde(~700gram) then i got just 25 gram amino alcohol!(total freebase was ~140gram but after gassing i just got 25 gram white powder)
Maybe benzylamine is impurity?
[Edited on 14-12-2012 by Waffles SS]
|
|
|
MrBlank1
Hazard to Self
 
Posts: 96
Registered: 5-2-2013
Location: Oz
Member Is Offline
Mood: Inadvertantly aloof?
|
|
I to have attempted this procedure, using n-methyl in DMSO solvent many times (Most recently with all lab grade reagent) . Upon gassing, a heavy
reddish-brown oil is the majority product, with a small amount of crystalline matter in suspension. Would salting out be the recommended path?
Also, I have reacted with just l-alanine, with and without dmso. No go with dmso (iirc). In all cases, dcm was a BIG help in cleaning the aqueous
layer. Most recent run, 4 litres of toluene was still coming off dirty with minimal lightening , 600 ml in dcm washes later, toluene wash clear.
[Edited on 5-2-2013 by MrBlank1]
[Edited on 5-2-2013 by MrBlank1]
|
|
|
MrBlank1
Hazard to Self
 
Posts: 96
Registered: 5-2-2013
Location: Oz
Member Is Offline
Mood: Inadvertantly aloof?
|
|
Alright, it's time to get some data...
In an effort to contribute some ( useful ) data myself to this thread, I'd like some opinions. Okay, here goes...
I currently have 4x 250mL of Benzaldehyde, 1000 grams of N-Methyl-DL-Alanine and 4000 mL of DMSO ( all 3 Lab grade with coa ), as well as 2x 1000
grams of L-Alanine ( Pharma grade ). I have plans to use 1x 250mL of the aldehyde to perform 2 runs with N-Methyl-DL-Alanine, 1 will be done neat (
ala Q.Doms procedure ) and one in DMSO ( ala Uncle Fester meets Q.Dom ), but more on that in a moment...
The rest, I'm open to suggestions / directions to gather more data for this thread. PLEASE NOTE : I am very lazy, and not interested in an end
product yield ( usually ), unless yield analysis is the purpose of the experiment. I have no problems doing (10+)x 6 hr refluxes in the next week, but
have little patience for wasting even 2 hours to extract all the end products, as I have no use for PPA, Ephedrine or Pseudoephedrine.
In short, I have more interest in the ' journey ', with end product being isolated in amounts only needed to confirm ' proof of concept ' ( usually
). I'm also more interested in seeing how the actual reaction varies with conditions. so for example, in the above procedure written by Q.Dom, once I
have my RXN mix, I would ( usually, and previously have, ) seperate off say 10/20%, then do the workup at 1/10 or 1/5 scale, and halve the expected
yield due to my sloppiness, and "other uncalculated" issues ( eg [ 35grams x 0.10 ] x 0.5 = 1.75grams ). Then I extrapolate the figures, dump it all,
and go again.
** PLEASE keep the above in mind, because whilst I am willing to work-up/isolate entirety of wanted product(s) and remaining
materials in the rest in the name of data, I just don't wanna  . ** . **
So again, I have the reagents, both hotplate/mag.stirrer and mantle, stark trap and condenser, vacuum system ( strong water pressure and 4
aspirators, but vac.gauge is brass threaded $20 cheapo ), 12v and 240v temp controllers, standard dist glassware, and capacity to work in 1L
quantities easily, 3 to 5 L with overhead stirring in oil bath, and ONLY if necessary, 5L filtration flask and 5L sep funnel ( both unused ). And I
dont care if i get any products.
Okay guys, your turn... Tell me what you want done, and how. If it will truly aid the ' flow of information ', I'm all in, no expectations other than
to learn and enjoy. I have a few days to prepare, so please let me know.
Getting lazy now, will give more details on my plans in next 12/24 hrs.
P.S. To Nicodem ( mostly ), plus Psychloknight, Quantum Dom and others that have given freely of their time, I offer my sincere gratitude, for the 5+
years that I have ' leeched ' info for myself, with-out contributing. You are the embodiment of 'freedom of information interchange', and I bow deeply
to you peeps.
If you guys want something done, say the word, this is the only way I can give back to this community. Sorry for the long winded post
- MrBlank1
[Edited on 4-3-2013 by MrBlank1]
|
|
|
psychronizer
Harmless

Posts: 6
Registered: 21-3-2013
Member Is Offline
Mood: No Mood
|
|
this is long thread and I dont have much the time to read it,seems to me you're trying to make phenylpropanolamine....the Henry reaction can be used
for this....usually a mixture of Benzaldehyde and nitroethane under the influence of a basic catalyst forms the nitro propenyl benzene because the
basic catalyst and reaction conditions (and the alpha hydrogens on nitroethane's alpha carbon are stroppy little buggers) force the molecule to
dehydrate-there is references out there that'll show you how to stop that from happening, so all you end up with is 1 phenyl 1 hydroxy 2 nitro
propane. the upshot of this is that there's no double bond to saturate, and that nitro can be reduced by any number of readily available simple
reductions. isnt that a whole bunch simpler than that other stuff in the thread? or am I skimming too fast and missing something vital?
|
|
|
Fluffy
Harmless

Posts: 1
Registered: 15-5-2013
Location: Sweden
Member Is Offline
Mood: No Mood
|
|
1) You are right that you get 1-phenyl-2- nitro-propane-1-ol if you condense nitroethane with bensaldehyde and use a strong base as NaOH instead of
the usually used weaker butylamine (or other alkylamine) as catalyst which here gives PNP (1-Phenyl 2-Nitro 1-Propene) instead.
This nitroalcohol can easily by reduced to PPA (LAH in THF). But this is a bulky 2-step reaction with all non-OTC and/or listed reactants!
2) What you miss is that this Akabori reaction is a very simple way to get racemic PPA from almost OTC reagents under very mild conditions by letting
Alanin decarboxylate (85C in DMSO) and almost simultanously react with bensaldehyde.
3) No reduction is necsesary here which makes it a very interesting and versatile reaction, possible to variate by using other aminoacids and/or
ringsubstituted bensaldehydes leading to a wide range of amino alcohols in one step.
[Edited on 16-5-2013 by Fluffy]
[Edited on 16-5-2013 by Fluffy]
[Edited on 16-5-2013 by Fluffy]
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
There are shitload of articles if you get them translated, they should point a LOT of people in the right direction. There is a reason you should use
racemic n-methylalanine, as no matter what you start with, you end up with a racemic product (the relevant carbon loses a hydrogen, gains a benzyl
group, then loses a carbon dioxide group - quarternary carbon stereobonds just don't cop that shit
Attachment: Akabori.Momotani.On.the.New.Reaction.Giving.Alcamines.of.Ephedrine.and.Adrenaline.Series.pdf (318kB)
This file has been downloaded 1297 times
Attachment: Akabori.Momotani.Uber.Eine.Neue.Bildunsweise.des.Ephedrins.und.der.Verwandten.Verbindungen.Zur.Biogenese.von.Ephedrin.un (357kB)
This file has been downloaded 1127 times
Attachment: Yokoyama.etal.Reaction.of.Free.Amino.Acids.with.Aldehydes.One.Step.Synthesis.of.Amino.Alcohol.Akabori.Rxn.pdf (91kB)
This file has been downloaded 1246 times
Fluffy - READ THE ARTICLES - there ain't no way you are getting PPA from this reaction, Ephedrine yes, PPA no way, alanine gives diphenylethanolamine.
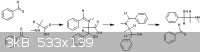
[Edited on 17-5-2013 by aliced25]
From a Knight of the Realm: "Animated movies are not just for kids, they're also for adults who do a lot of drugs." Sir Paul McCartney
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
This is from a series dealing with the Akabori reaction, 7 papers let's see if they load. These papers are ALL in Japanese and badly need to be
translated - the aldol condensation, fuck, all of 'em.
Attachment: 1.Synthesis.of.Chloramphenicol.by.Akaboris.Reaction.pdf (455kB)
This file has been downloaded 796 times
Attachment: 2.Decarboxylation.of.Amino.Acids.in.Various.Solvents.pdf (570kB)
This file has been downloaded 955 times
Attachment: 3.On.the.Condensation.Products.of.B.Hydroxy.a.AminoAcids.with.Formaldehyde.pdf (385kB)
This file has been downloaded 800 times
Attachment: 4.Decarboxylation.of.the.Schiffs.Base.of.Free.Amino.Acid.pdf (500kB)
This file has been downloaded 885 times
Attachment: 5.Aldol.Condensation.of.Amino.Acids.with.Aldehydes.pdf (476kB)
This file has been downloaded 1007 times
Attachment: 6.Decarboxylation.of.N.Methyl.AminoAcid.by.Aromatic.Aldehyde.pdf (213kB)
This file has been downloaded 956 times
Attachment: 7.Decarboxylation.of.N.Acyl.AminoAcids.with.Aldehydes.A.New.Reaction.of.Oxazole.Ring.Formation.pdf (312kB)
This file has been downloaded 793 times
From a Knight of the Realm: "Animated movies are not just for kids, they're also for adults who do a lot of drugs." Sir Paul McCartney
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Another 7 paper series, in Japanese but with some English - describes the product of alanine + benzaldehyde as diphenylethanolamine, go figure.*
* Although from memory, they are useful somewhere, local anaesthetics come to mind, either that or something...
Attachment: 1.New.Facts.on.Akabori.Reaction.pdf (1022kB)
This file has been downloaded 1171 times
Attachment: 2.A.New.Reaction.giving.Alkamines.and.12.Diphenylethanolamine.Series.pdf (848kB)
This file has been downloaded 1170 times
Attachment: 3.A.New.Reaction.giving.Alkamines.and.12.Diphenylethanolamine.Series.2.pdf (752kB)
This file has been downloaded 746 times
Attachment: 4.Attempts.to.Access.Optically.Active.Products.pdf (920kB)
This file has been downloaded 862 times
Attachment: 5.Results.Obtained.by.Paper.Chromatography.pdf (539kB)
This file has been downloaded 1132 times
Attachment: 6.Untitled.pdf (746kB)
This file has been downloaded 881 times
Attachment: 7.Untitled.pdf (564kB)
This file has been downloaded 861 times
From a Knight of the Realm: "Animated movies are not just for kids, they're also for adults who do a lot of drugs." Sir Paul McCartney
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
MrBlank1 - if you have N-methylalanine I would strongly suggest you read the abstract (in English) of the second series of papers (For anyone else,
pyruvic acid isn't watched, reductive amination of it with aluminium/amalgam will give n-methyalanine). I would suggest using a translator program to
try and get the gist of what they are saying with regard to temperatures and reaction conditions and follow them if possible. I have posted the
original Akabori & Momotani papers, they used pyridine which is apparently unnecessary. One is in German and should be a fucking cinch to get
translated on this site.
From a Knight of the Realm: "Animated movies are not just for kids, they're also for adults who do a lot of drugs." Sir Paul McCartney
|
|
|
pHi
Harmless

Posts: 1
Registered: 21-6-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aliced25  |
Fluffy - READ THE ARTICLES - there ain't no way you are getting PPA from this reaction, Ephedrine yes, PPA no way, alanine gives diphenylethanolamine.
|
Quote: Originally posted by Quantum_Dom  |
1-Lots of people are arguing that the only compound obtained in this procedure is 1,2-diphenylethanolamine (DPEA) quoting this study and affirming that no PPA is obtained at all. DPEA is indeed the major product but it is easily removed in the work-up. The reason the
authors are not isolating any PPA is mainly due to 1) the very little scale theyre working on (amount of PPA is almost insignificant at this scale),
2) they are omitting to concentrate the aqueous phase extracts in the work-up (the latter contains PPA*HCl). This will be re-explained later and will
prove by the same occasion that referring to that article, to conclude that PPA is not in fact produced, is incorrect.
|
Quantum_Dom is the OP and makes the above statement in his opening post. He reiterates several times later in the the thread that PPA is not the main
product of the reaction but is produced as a secondary product. DPEA is washed from the aqueous layer with DCM (or chloroform) and then PPA is worked
up after that.
Are you refuting that PPA is produced at all? All of your attached articles are in Japanese which I am unable to read. Given that you request a
translation of the articles yourself, it's a little confusing as to why you would make such a statement "PPA no way".
|
|
|
tadpoleannihilater
Harmless

Posts: 7
Registered: 3-10-2013
Member Is Offline
Mood: No Mood
|
|
Would it be possible to skip the removal of DPEA with DCM by fractional distillation?
Also would it be possible to use diethyl ether or n-pentane instead of toluene?
I will try and find out the hard way. 
[Edited on 5-10-2013 by tadpoleannihilater]
|
|
|
| Pages:
1
..
3
4
5
6 |
|