IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Azo group and Cinnamic acid substitution orientation
Hey guys,
In the book COPAE, Chapter IV, in the substitution order rule 1, "The orienting effect", it says that Azo groups (-N=N-) orient ortho/para
positions and Cinnamic acid (-CH=CH-CO-) orient ortho/para aswell. However in the induction (the method I always use to determine activating
and desactivating groups) I would have the following:
-N+=N--R (unless the R was a really electronegative element like Fluor or Oxygen)
-C+H=C-H-C+O-
And what I would expect from those groups is to induce the aromatic nucleos in a way it would yield meta substitutions. Are the charges there
wrong or they are some kind of exception to the rule? I'm banging my head here to understand it.
The other exception they show is the -CCl3, but using the induction method it does yield meta, which is what the book says.
Any clues why does that happen? Is that method of determining the activating/desactivating groups wrong?
Thanks in advance 
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Activation/deactivation can take place via resonance as well as inductive effects. Even though there may be electron withdrawing by inductive effect
which would lead to a meta director, the pi electrons induce resonance which causes an electron donating effect which overpowers the inductive
electron withdrawing leading to ortho/para substitution.
For example the -OH is electron donating and ortho/para directing overall because it is electron donating by resonance despite the slight electron
withdrawing due to induction.
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
I just realized that now, -OH despite being electron donating is a ortho/para director in Picric acid for example.. Using the inductive effects it
should be meta director.
I guess I understood, but I'm finding a little hard to visualize how the resonance in the structure would lead to ortho/para directing. I'll try to
look further for information about this.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
The top shows all resonance forms of intermediates resulting from ortho attack. The second and third show the same for meta and para attack
respectively. The blue resonance structures are resonance structures possible only for ortho and para attack in this case. The additional resonance
structure adds stability and makes these more favorable attacks in this instance.

|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Thanks a lot crazyboy!
I now understand why those groups despite being electrons withdrawing are ortho/para directors. They probably just like the -OH group have some extra
ressonance structures, giving them more stability on ortho and para positions.
So the best way to know if a group is an activating group or desactivating one is actually to memorize them? Guess it would be really complicated to
imagine all the ressonance structures from all the groups.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
http://www.learnchem.net/orgo/aro2_nom.shtml
This list is pretty comprehensive. All deactivators with the exception of halogens are meta directors. All activators are ortho/para directors.
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Thanks for the link.
Sorry to insist on the subject although you already told how it works. But I guess I didn't have a good explanation on "Organic Chemistry I" about
this, and the fact that I had to study by myself might have lead me to misinformation.
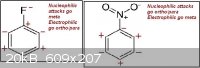
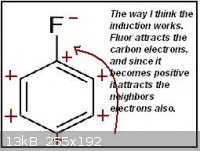
Until now I only used the induction to identify the meta and ortho/para directors group, as in the first attachment.
Thinking further I realized it doesn't make much sense the way those neighbors carbons are affected. I think that a negative charged carbon would
actually make the neighbors slightly negative, the same for positive charged carbons, just like in the second attachment.
After your explanations (all very easily to understand) it seens to me that its actually more about the carbocation structure being stabilized than
the inductive charges itself. Which would make more sense for those exceptions.
That inductive method I used is then completely wrong? Are the charges in the first and/or second attachments wrong? So the charge is actually not
that important, and the intermediate structure stabilization is the key to find which groups are meta and ortho/para directors?
Sorry again to bother you with more questions, I'm just kind of confused, trying to fit all the information together and to get rid of the misleading
ones. And thanks for all the help so far.
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Just deconsider the last post, as I thought the first attachment is completely wrong. I'm feeling even a little stupid for having not realized that
before.
Thanks for the help Crazyboy
|
|
|