| Pages:
1
2 |
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
New Compound
I believe I have synthesized a new compound. How should I go about the next step?
Is this a big accomplishment, synthesizing a new compound?
My compound is attached if you do not believe it is truly a new compound!
[Edited on 31-10-2012 by Magelia]
[Edited on 31-10-2012 by Magelia]
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Compound attached.
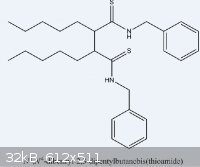
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
First, name the compound (using IUPAC nomenclature, of course), then do a SciFinder search or similar to see if it has been reported earlier. Also,
provide an NMR spectrum so you can be sure that it's the compound you're speaking of.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Yes, it is the compound. I had the NMR expert look into it, and it is indeed the dimer.
I did do a search on scifinder and found nothing.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2758
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Now you buy donuts for the research team. That is what we always did when we achieved a major goal for the team, like a publication or novel
molecule.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Ok, if it's new then congrats.
What is it good for? Is there any similar like substances, or does it have any idea that what could it be used in?
If the answer for the above questions is yes, then write an article, make 4-5 similar comounds and send it to a chemical journal. If they also think
that this is good for something, then they may publish it.
P.S.: today I have made 2 new compounds 
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Ephesian
Hazard to Self
 
Posts: 97
Registered: 14-8-2012
Member Is Offline
Mood: No Mood
|
|
turn it into a salt, get a crystal structure, bam! publication
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Ok thanks!
Could anyone double check in their database that my compound is actually a "new" compound if you guys don't mind? 
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
A quick structure search in SciFinder turned up no hits, indicating it's probably unknown.
If you don't mind my asking, how did you make this? From your writing, it sounds like you dimerised the parent N-benzyl thioamide. I was working on
this type of chemistry a while back, it was pretty annoying (and even worse for a co-worker, who spent really significant amounts of time on it!). We
had some really ugly thioamides, though, which didn't help (and, of course, half the irritation comes from having to make the thioamides themselves!).
|
|
|
trip96
Harmless

Posts: 16
Registered: 23-7-2012
Member Is Offline
Mood: No Mood
|
|
Hey guys! I have some super basic questions. I hope these aren't too simple.
1. How are you making these new compounds?
2. How do you confirm that they are the compounds?
3. Why are you making them?
Sorry if this is annoying to answer or anything like that. I'm very interested in this topic though and would like a short walk through to get up to
speed.
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What is so special about making new compounds? I am quite sure that I also can make new compounds which never have been made before. It is like
playing with big people's LEGO which allows you to make new objects from small bricks which have never been made before.
Making a new compound in itself is not that spectacular, you have to explain what is special about your compound. Does the synthetic procedure for
making it add something new to the science of chemistry (e.g. some not yet known kind of reaction or a very smart and uncommon combination of already
known reactions)? Has the newly made compound special properties which really set it apart from other compounds?
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Yah, it's weird... anyone know the possible mechanism to which my above compound dimerized?
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I've made compounds new to chemistry during undergraduate research...just juggle around a few functional groups and you have scores of them. That
doesn't make them extremely exciting, though it is cool.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
One name of your compound would be
N,N'-dibenzyl-dodecyl-6,7-thioamide
[Edited on 6-11-2012 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Thanks for the reply! Still trying to figure out what is going on!
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
That would be a name for it, but it wouldn't be a correct name for it.
The molecule has two thioamide groups, that name has only one
|
|
|
gutter_ca
Hazard to Others
  
Posts: 173
Registered: 7-6-2010
Location: California
Member Is Offline
Mood: Bored at work!
|
|
Quote: Originally posted by unionised  |
That would be a name for it, but it wouldn't be a correct name for it.
The molecule has two thioamide groups, that name has only one |
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
(N,N'-dibenzyl)-Dodecane,6-7-bisthiocarboxamide perhaps. Probably not IUPAC, but intelligible.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Anyone know how I can draw the mechanism of its formation from its mono part? Mechanism of the formation of the dimer?
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
We always thought it went via enolisation, followed by oxidative coupling to the disulfide and then a (3,3)-sigmatropic rearrangement (like a Cope or
Claisen rearrangement) to give the dimer.
There's a bit of literature out there on this stuff, which supports that mechanism.
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Could you perhaps let me know if my mechanism drawn below is correct?
I tried looking for a mechanism in literature but wasn't able to find one.

[Edited on 7-11-2012 by Magelia]
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Never ever seen such reaction.
The S-S bond could form if vou oxidize it a bit, but I'm a bit unsure that wit will work with thioamides... Also the isomerisation and the C-C bond
forming is also a really strange thing there...
Do you have any else data except the NMR? An MS? Also could you post the reaction conditions? This sketch reaction mechanism looks really weird with
this isomerisation and condensation....
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Yup, that's pretty much it. As kristofvagyok says, it should need an oxidant to form the disulfide, but we saw cases where there was no obvious
oxidant (and that includes dissolved oxygen, which we removed from the solvents), so it's possible that something else is going on, too.
There is, as I said, some good literature on this - mostly intramolecular reactions, but I believe some examples of intermolecular (and we certainly
got some intermolecular ones to work, just never reliably enough to be useful). I think the relevant stuff goes back to the 70s, at least. I'm afraid
it wasn't a core component of my work, however, so I'm not as up on those details as my colleague.
|
|
|
Magelia
Harmless

Posts: 28
Registered: 26-10-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kristofvagyok  |
Never ever seen such reaction.
Do you have any else data except the NMR? An MS? Also could you post the reaction conditions? This sketch reaction mechanism looks really weird with
this isomerisation and condensation.... |
No, I do not have a MS. Reaction conditions: Heptyne + Benzyl amine + sulfur.
Ever hear of such a rxn?
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I have heard about that reaction, that is not the tricky part. The question would be that how do you know that the thioamide forms a dimer with a
reaction mechanism that you have described above? That is the part what is a bit wierd to me.
But I will check it on Monday at the library.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
| Pages:
1
2 |