| Pages:
1
2 |
rstar
Hazard to Others
  
Posts: 138
Registered: 22-9-2011
Location: Besides valence shell
Member Is Offline
Mood: Dark
|
|
red muriate of potash ?!
I went to a fertilizer shop today, and saw a bag named "Potash". It was also written on the bag "Contains Muriate of Potash" .
I thought it was KCl, but inside the bag there was reddish powder. I told he shopkeeper to gimme a half kilo of that stuff.
Well now wat is that red stuff ?
i also tried to dissolve it in tap water, but the red particles didnt dissolve, and i filtered the solution. I believe it contains KCl.
and
how to test if the solution contains KCl ?
"A tidy laboratory means a lazy chemist "
- Jöns Jacob Berzelius
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
If you have a soluble silver salt, mixing the two together should produce the insoluble, tan precipitate of AgCl.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Sure? IIRC AgCl is white, AgBr more yellow/tan and AgI the most intensely coloured.
http://www.chemguide.co.uk/inorganic/group7/testing.html
Your red stuff could well be an iron salt, possibly the oxide.
[Edited on 18-10-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
It was always referred to as 'tan' whenever discussed, maybe something about hydration changing the color? Dry AgCl probably looks white.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
rstar
Hazard to Others
  
Posts: 138
Registered: 22-9-2011
Location: Besides valence shell
Member Is Offline
Mood: Dark
|
|
i hv no silver or lead salts 
and yea, i believe that red salt to be Iron oxide
[Edited on 18-10-2012 by rstar]
"A tidy laboratory means a lazy chemist "
- Jöns Jacob Berzelius
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Alternative ways to test if the solution contains any soluble compounds are to boil it down or evaporate to the point where whatever might be
dissolved drops out of solution (due to saturation). So take a lot of the red stuff, and pour boiling water over it, and stir for a bit. Filter out
while still hot and this should give you a fairly saturated solution of whatever's in there.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
interestingly, when i leach wood ashes to extract potash, i get a red solution, also. i thought the colour was from something like iron or manganese.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
AgCl is white like snow. The wet material as precipitated is white and the dry solid also is white. In
bright light, however, AgCl slowly turns purplish grey, due to decomposition and subsequent formation of very small amounts of metallic Ag. This
change in the material is the basis of photography.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
"Why is some MOP red and some white? Both red and white MOP come from the same evaporite ore deposits. The ore usually contains sodium chloride
(common table salt) and other impurities. Naturally occurring MOP ore has a reddish color due to minor amounts of iron impurities. The color
difference in the final fertilizer product is due to different methods of recovery and processing."
http://www.ipni.net/ppiweb/agbrief.nsf/5a4b8be72a35cd4685256...
|
|
|
feacetech
Hazard to Others
  
Posts: 163
Registered: 12-2-2007
Member Is Offline
Mood: No Mood
|
|
They add a colouring agent to murate of potash so that it doesnt get confused with another product when they despatch it, I cant remember which but
its another white crsytaline salt
The Granular potash I see ranges from 88-99.5% KCl with a average of 94.5% KCl
|
|
|
platedish29
Hazard to Self
 
Posts: 76
Registered: 2-9-2012
Member Is Offline
Mood: absorbing CO2
|
|
Most PbCl2 produced via commercial grade chemicals contains molibdenium, which tarnishes stuff.
If it is not AgCl it can be Mo.
|
|
|
feacetech
Hazard to Others
  
Posts: 163
Registered: 12-2-2007
Member Is Offline
Mood: No Mood
|
|
this maybe the agent they use REDON-50
http://www.ecplaza.net/trade-leads-seller/colouring-agent-fo...
some sources of poatsh can contain contaiminats from extraction/processing of 0.05% Fe or other metal oxides
but normaly it has the colouring agent added to it nowdays as for the reason in the above post.
Alternativly they could be colouring it becuase people are use to it being pink/red from the gold old days
[Edited on 19-10-2012 by feacetech]
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i know which stuff that is, i've gotten about 8 lbs. of that red potassium chloride and it turns white as table salt when you convert it to the
chlorate. i dissolve a whole bunch in a stew pot and warm it up on the stove.warm potassium chloride like that is easier to filter and all the red
clay looking stuff will be left on the filter. i thought it was silvite or something raw from a mine somewhere in texas but after the salt has
recrystalized it will turn white. even the left over reddish water that doesnt recrystalize makes pure white potassium chlorate. actually if you
recrystalize the salt to purify it further,the coffee filters themselves will burn like gun powder when dried and ignited. i love the stuff and its
dirt cheap too about 4lbs. for five bucks.
[Edited on 19-10-2012 by cyanureeves]
[Edited on 19-10-2012 by cyanureeves]
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
I haven't seen this stuff since I was a kid. The red powder filtered out real easily, and a hot concentrated solution gave beautiful needle-like
crystals when it cooled.
|
|
|
rstar
Hazard to Others
  
Posts: 138
Registered: 22-9-2011
Location: Besides valence shell
Member Is Offline
Mood: Dark
|
|
This is wat the red residue on the filter paper looked like, after drying
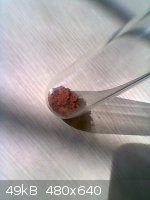
i believe it to be iron oxide. Any tests for it ?
Also there were small needle like crystals on the dried residue, but its hard to see here
[Edited on 19-10-2012 by rstar]
"A tidy laboratory means a lazy chemist "
- Jöns Jacob Berzelius
|
|
|
rstar
Hazard to Others
  
Posts: 138
Registered: 22-9-2011
Location: Besides valence shell
Member Is Offline
Mood: Dark
|
|
any tests to know if that red stuff is iron oxide ?
(pls dont tell me to add HCl, i'm out of it  ) )
"A tidy laboratory means a lazy chemist "
- Jöns Jacob Berzelius
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
Fe(III) in solution gives prussian blue with ferrocyanide.
|
|
|
rstar
Hazard to Others
  
Posts: 138
Registered: 22-9-2011
Location: Besides valence shell
Member Is Offline
Mood: Dark
|
|
sorry, no acess to ferrocyanide. 
will it work:
adding NaOH to Fe3+ solution ?
"A tidy laboratory means a lazy chemist "
- Jöns Jacob Berzelius
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
you can make the prussian blue also by strongly heating the iron oxide with some nitrogen and carbon.
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
sounds simple enough..let me get my jar of powdered nitrogen =D
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Sylvite fun facts and photos.
"Sylvite is one of the last evaporite minerals to precipitate out of solution. As such, it is only found in very dry saline areas. Its principal use
is as a potassium fertilizer. Sylvite is found in many evaporite deposits worldwide. Massive bedded deposits occur in New Mexico and western Texas,
and in Utah in the US, but the largest world source is in Saskatchewan, Canada. The vast deposits in Saskatchewan, Canada were formed by the
evaporation of a Devonian seaway. Sylvite is the official mineral of Saskatchewan."
"Sylvite was first described in 1832 at Mt. Vesuvius near Napoli in Italy and named for the Dutch chemist, François Sylvius de le Boe (1614–1672)."
http://en.wikipedia.org/wiki/Sylvite
Production
"Deeply buried potash deposits are found throughout the world. The dominant mineral is sylvite (KCl) mixed with halite
(sodium chloride), which forms a mixed mineral called sylvinite. Most K minerals are harvested from ancient marine deposits
deep beneath the Earth’s surface. They are then transported to a processing facility where the ore is crushed and the K salts
are separated from the sodium salts. The color of KCl can vary from red to white, depending on the source of the sylvinite
ore. The reddish tint comes from trace amounts of iron oxide. There are no agronomic differences between the red and white
forms of KCI."
"Some KCl is produced by injecting hot water deep
into the ground to dissolve the soluble sylvinite mineral
and then pumping the brine back to the surface
where the water is evaporated. Solar evaporation is
used to recover valuable potash salts from brine water
in the Dead Sea and the Great Salt Lake (Utah)."
http://www.ipni.net/publication/nss.nsf/0/8FBD66599EAB433F85...
[Edited on 23-10-2012 by Morgan]
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
if you use excess carbon,
a limited supply of air will suffice as the source of nitrogen.
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
have you tried this yourself successfully? or can you link a reference? i'm pretty sure the "source of nitrogen" has to be more consistent, such as
protein matter etc, in order to synthesize prussian blue as in the old days:
http://www.sciencemadness.org/talk/viewthread.php?tid=21329
btw the OP was looking for a test for iron (oxide)..i doubt your method is of any analytical relevance.
[Edited on 23-10-2012 by tetrahedron]
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by tetrahedron  |
have you tried this yourself successfully? or can you link a reference? i'm pretty sure the "source of nitrogen" has to be more consistent, such as
protein matter etc, in order to synthesize prussian blue as in the old days:
http://www.sciencemadness.org/talk/viewthread.php?tid=21329
btw the OP was looking for a test for iron (oxide)..i doubt your method is of any analytical relevance.
[Edited on 23-10-2012 by tetrahedron] |
i have done this two ways:
1) during welding of some mild steel, it developed a blue finish;
2) a couple of steel tuna cans, of different diameter, arranged to make a crude crucible with lid, were placed on my electric stove with a little
charcoal in them, and a cover fitted for the heating element to trap the heat. this was given about 20 minutes on high, after which some blue surface
developed inside
also, last night i examined a metal chimney and found some blue had formed on its outer surface (along with some rust). now i say that's a positive
qualitative test for iron, don't you?
the chimney was not magnetically attracted, so i guessed that it is stainless steel.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ElectroWin  | during welding of some mild steel, it developed a blue finish;
[...]
also, last night i examined a metal chimney and found some blue had formed on its outer surface (along with some rust). now i say that's a positive
qualitative test for iron, don't you? |
The blue sheen you're seeing sounds much more like thin-film
diffraction that it does like ferrocyanide.
|
|
|
| Pages:
1
2 |