| Pages:
1
..
18
19
20
21
22
..
30 |
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
....the hydrolysis of phenylacetamide will produce PAA, and a by product of an amine.... will the amine be methylamine?or ammonium chloride if
hydrolysed with HCL or ammonium sulfate if hydrolysed with sulfuric acid? .. once the phenylacetamide has been put into solution in methanol and a
strong solution of an acid or a base is added and refluxed for three hours...... does anyone have first hand notes on the hydrolysis, ....bits and
pices of the info was gathered at organic synthesis and reading Marchs 6th edition .....solo
[Edited on 9-8-2012 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by DJF90  | I've personally rotavapped some formalin to the point that it becomes much more viscous and begins to cloud. It's poured out on a hard surface in a
thin layer (read: pyrex baking dish) and as it cools it solidifies. Scraping this solid into flakes or "shavings" will allow proper desiccation over
anhydrous calcium chloride. The solid is removed and ground in a pestle and mortar before being returned to the desicator. The fine, dry powder that
is obtained is paraformaldehyde, (CH2O)n.
|
Interesting, so do you mean to say that the CH2O would "rather" polymerize than evaporate?
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Will the Bucherer reaction displace a hydroxy by an amino group in an alkyl.....?..or only on a benzene ring?..solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by solo  | | Will the Bucherer reaction displace a hydroxy by an amino group in an alkyl.....?..or only on a benzene ring?..solo |
The mechanism is depicted on the Wikipedia entry for Bucherer reaction. You can now answer your own question.
Also, the scope of this reaction does not expand to just any phenol. It has to be a phenolic compound that has a substantial equilibrium for the
reverse enolization.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Nicodem.....thanks for the reply, I had read,
"Replacement of hydroxy by amino groups occurs in the Bucherer reaction; the reagent is an aqueous solution of bisulfite and ammonia or an amine, and
the hydroxy groups of naphthols or resorcinol are most easily replaced. The Bucherer reaction occurs via the keto tautomers of the phenols (163), and
is not to be classed as aromatic substitution.
The 4-hydroxy group in 2,4-dihydroxyquinoline may be replaced by amines; hydrochloric acid aids the reaction with aniline (138). Aminolysis of an
o-nitro- phenol has been reported, but the reaction is more difficult than that of the corresponding nitrophenyl alkyl ethers (540). Here again it
might be argued that these direct replacements of hydroxy groups are in reality condensations of the keto tautomers of the phenols."
......source,
Aromatic Nucleophilic Substitution Reactions- Bunnett and Zahler
http://filecloud.io/nd8lh7eo
Ref.163-DRAKEO: Organic Reactions, Vol. I, p. 105. John Wiley and Sons, Inc., New York (1942).....this is located in the SM library
.....but i guess wanted for it to work on an alkyl chain....hence, my stubborn question...which is now answered ....thanks....solo
[Edited on 14-8-2012 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
The preparation of tetramine copper (II) sulfate is usually carried out with concentrated ammonia solution.
Can it be done by using household ammonia, with the quantities adjusted accordingly?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by Hexavalent  | The preparation of tetramine copper (II) sulfate is usually carried out with concentrated ammonia solution.
Can it be done by using household ammonia, with the quantities adjusted accordingly? |
Yes but if you want crystals of the tetramine copper II sulphate of it You will find 28% ammonia more suitable.
I never asked for this.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Why? Is it just because there will be less water present with the concentrated ammonia? My plan was just to add enough ammonia solution to aqueous
copper sulfate to form copper hydroxide and then to complex the precipitate again, before adding ethanol to precipitate out the tetramine copper
sulfate.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Can N-ethylhydroxylamine react with methyllithium to produce ethylmethylamine. if it works, then ethyllithium could be used for
diethylamine(ethylethylamine) and propyllithuim could be used for ethylpropylamine. Will it work?
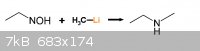
[Edited on 9-9-2012 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Would liquid sulphur, heated a bit above its melting point (~115 °C), attack food grade silicone polymer? Would vulcanization occur and affect it? I
have an idea, but I'm not willing to do any experiments yet.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 283
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
A couple of years ago I tried some hydroforming with my pressure washer but it didn't have enough pressure. It says 1800 PSI on it, but it's old so
I'm sure it's less than that. Anyone know of a cheap and readily available pump (automotive maybe?) that can get to pressures of say 2500 PSI and
higher? If I can find the right pump I'd like to give this another try. I'm trying to form 4" diameter 1.6mm thick copper circles into a conical
cavity. Considering how incredibly soft copper is in it's annealed state, I thought my pressure washer would be plenty adequate. I thought wrong 
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  | | Would liquid sulphur, heated a bit above its melting point (~115 °C), attack food grade silicone polymer? Would vulcanization occur and affect it? I
have an idea, but I'm not willing to do any experiments yet. |
The experiment to do is to immerse some samples
of the silicone you want to use in liquid sulfur and pull them out an periodic intervals. Wash in toluene to clean them. Examine, with a magnifier if
needed.
I would imagine that you'll get an attack. What I don't know is the degradation rate. I've not done the experiment, but I imagine you'll get
liberation of H2S and dehydrogenation of the polymer.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by watson.fawkes  | Quote: Originally posted by Endimion17  | | Would liquid sulphur, heated a bit above its melting point (~115 °C), attack food grade silicone polymer? Would vulcanization occur and affect it? I
have an idea, but I'm not willing to do any experiments yet. |
The experiment to do is to immerse some samples
of the silicone you want to use in liquid sulfur and pull them out an periodic intervals. Wash in toluene to clean them. Examine, with a magnifier if
needed.
I would imagine that you'll get an attack. What I don't know is the degradation rate. I've not done the experiment, but I imagine you'll get
liberation of H2S and dehydrogenation of the polymer. |
Thanks. I've got only one piece of it and I don't want to spend money on this experiment. Plus I don't have the opportunity to do it soon...
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Can 4A molecular sieves be used to remove water vapor from H2 gas? Sigma says 3A is suited to this purpose but I only have 4A. Do molecular sieves
even absorb enough water to make them viable for drying H2 mixed with steam or would I just be better off passing the hydrogen over sodium sulfate or
a similar chemical dessicant?
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gnitseretni  | A couple of years ago I tried some hydroforming with my pressure washer but it didn't have enough pressure. It says 1800 PSI on it, but it's old so
I'm sure it's less than that. Anyone know of a cheap and readily available pump (automotive maybe?) that can get to pressures of say 2500 PSI and
higher? If I can find the right pump I'd like to give this another try. I'm trying to form 4" diameter 1.6mm thick copper circles into a conical
cavity. Considering how incredibly soft copper is in it's annealed state, I thought my pressure washer would be plenty adequate. I thought wrong  |
Seems like you'd need a hydraulic pump of some sort, and they don't really come cheap. Even a hand-operated pump (which would be OK for your purpose
since you don't need any significant flow) sells for over $100.
The less you bet, the more you lose when you win.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 283
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
Ha, I just bought a hand operated pump for $70 five mins ago. Goes up to 9700 PSI. Should be enough 
|
|
|
mineralman
Hazard to Self
 
Posts: 64
Registered: 15-6-2012
Location: WALES UK
Member Is Offline
Mood: complicated
|
|
I can't find any answer and hope it's new.
Can the ice build up from a freezer be classed as "UNCONVENTIONAL" distilled water when melted? mm
|
|
|
mineralman
Hazard to Self
 
Posts: 64
Registered: 15-6-2012
Location: WALES UK
Member Is Offline
Mood: complicated
|
|
Quote: Originally posted by gnitseretni  | A couple of years ago I tried some hydroforming with my pressure washer but it didn't have enough pressure. It says 1800 PSI on it, but it's old so
I'm sure it's less than that. Anyone know of a cheap and readily available pump (automotive maybe?) that can get to pressures of say 2500 PSI and
higher? If I can find the right pump I'd like to give this another try. I'm trying to form 4" diameter 1.6mm thick copper circles into a conical
cavity. Considering how incredibly soft copper is in it's annealed state, I thought my pressure washer would be plenty adequate. I thought wrong  |
You can get vac pressure flow connections for air compressors, there may be one for water aplications. Add a water cooling system to aid in getting
higher pressure's. maybe the pressure washer you already have can get you there. just a thaught. MM
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Quote: Originally posted by crazyboy  | | Can 4A molecular sieves be used to remove water vapor from H2 gas? Sigma says 3A is suited to this purpose but I only have 4A. Do molecular sieves
even absorb enough water to make them viable for drying H2 mixed with steam or would I just be better off passing the hydrogen over sodium sulfate or
a similar chemical dessicant? |
4A MS will absorb water, if they are well activated (usually bought that way, but if old, they can be heated under vacuum to dry them.) You could
also use a chemical desicant like NaSO4, although it is slow to absorb, but it will be OK if there is a lot of H2O. But these types of drying agents
can only do so much, so fast, so the H2 needs to be relatively dry when it starts to have them dry it well. If you have very wet H2, it may take
several drying steps/towers to dry it well. But even H2 generated from water hydrolysis can be dried sufficiently with a drying column or two, under
most cases. We have used hydrolysis H2 generators for H2 in the lab, and they worked fine.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
A bubbler
through concentrated sulfuric acid seems to be the standard first element in any drying train for gases generated from aqueous solutions. It will
eventually dilute, of course, and lose its drying power.
Also, the reference I have on hydrogen brazing recommends 5A sieves for taking water vapor from tank hydrogen. My guess is that the adsorption rate
may be a bit higher that with 4A.
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
making sodium fluoride
can calcium fluoride be converted to sodium fluoride by boiling with soda, akin to the method starting with barium sulfate?
http://www.sciencemadness.org/talk/viewthread.php?tid=9123
CaF2 is even more soluble than BaSO4..
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by solo  | ....the hydrolysis of phenylacetamide will produce PAA, and a by product of an amine.... will the amine be methylamine?or ammonium chloride if
hydrolysed with HCL or ammonium sulfate if hydrolysed with sulfuric acid? .. once the phenylacetamide has been put into solution in methanol and a
strong solution of an acid or a base is added and refluxed for three hours...... does anyone have first hand notes on the hydrolysis, ....bits and
pices of the info was gathered at organic synthesis and reading Marchs 6th edition .....solo
[Edited on 9-8-2012 by solo] |
Vogel 3rd ed uses 30% caustic refluxed for 30 mins, reaction followed via NH3 coming off (ie past the condenser), when this ceases then finished. Also
same edition uses refluxing HCl, reaction completed when solution loses turbidity.
if you have time i suggest just leaving it in 30%hcl for a week.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
If I prepare methyl benzoate with methanol and benzoic acid I will get that great fruity smell that we all know and love, but, if I prepare benzyl
methanoate, i.e. with benzyl alcohol and methanoic acid (formic acid), will it still have the same smell?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Hexavalent  | | If I prepare methyl benzoate with methanol and benzoic acid I will get that great fruity smell that we all know and love, but, if I prepare benzyl
methanoate, i.e. with benzyl alcohol and methanoic acid (formic acid), will it still have the same smell? |
Why would it...the molecule is a different shape.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
That was what I thought...although I wasn't sure as the aromatic ring and aliphatic substituent were in the same 'place'.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
| Pages:
1
..
18
19
20
21
22
..
30 |