| Pages:
1
2 |
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
Trying to seperate MnCl2 & KCl from dead reaction mixture
Hello!
So I've made some a gas generator which produces Cl2 :
2KMnO4 + 16HCl --> 2KCl + 2MnCl2 + 8H2O + 5Cl2
(158g) (36,4g) (149g) (394g) (144g) (355g)
The chlorine gas was passed through a hot NaOH solution to make sodium chlorate. BUT some of the chlorine gas is ofcourse dissolved in the 144ml of
water so also keep that in mind.
So we got 144ml of water (with Cl2) + 394g MnCl2 and 149g KCl.
My question is , how to seperate the MnCl2 from the KCl?
First I was thinking of exchanging the ions of the potassium chloride, like adding sodium chlorate to the mixture which would produce Potassium
chlorate which isn't that good soluble in water so we could get the potassium chlorate out but then we still got leftover potassium chlorate dissolved
in the water + some NaCl because that was also formed...
Maybe some kind of electrolysis then? So I don't know actually, anyone knows a good way to seperate them? I got basic chemicals and decent basic lab
setup (no distil. set).
Another way could be by adding silver nitrate to the reaction mixture which would also react with the potassium chloride (KCl + AgNO3 => KNO3 +
AgCl) and due silver nitrate has a very good solubilty in water but silver nitrate is way to expensive and I don't want to waste it.
[Edited on 7-7-2012 by CrEaTiVePyroScience]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Precipitate it using carbonate or hydroxide ions, and then pyrolize it to get the dioxide that you can use again as an oxidant for chloride anions.
Sodium salts are the cheapest. Beware - you'll never get rid of the sodium, which will be obvious from the flame test. Therefore, use the recycling
for batches that you produce chlorine with.
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
Possible to give some more details of your proces?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Just dump the carbonate (sodium carbonate is the cheapest) solution into the spent mixture and mix it thoroughly. Knowing how much manganese(II) is
inside would help a lot to avoid wasting the carbonate or throwing away the manganese solution.
Wash it with plenty of tap water and then rinse two or three times with distilled water. A vacuum technique with the Büchner funnel would be
very helpful unless you prefer several days of annoying gravity filtration. If I had it while doing the same thing few years ago, I
would reduce it to 15 minutes of work.
If you have to resort to the gravity filtration, you should wash the precipitate by putting it into the beaker again, and swirling it. Büchner
funnels allow washing in situ.
All that's left is to heat it for several minutes above 200 °C. I've got a chipped beaker I use instead of expensive crucibles. You're left with a
fine powder of the dioxide.
Be careful with manganese. Buy a dust mask.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Or precipitate as MnCO3, wash and dry. Then redissolve in in HCl.
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
It doesn't have to be MnCO3 just seperating MnCl2 OR KCl is also fine for me , if possible.
I already know two ways of getting potassium chlorate and potassium nitrate from the mixture
( KCl + NaClO3 (which is added) => KClO3 + NaCl ) adding NaClO3 will make KClO3 will precipitate out (http://www.youtube.com/watch?v=0pdt8GidpGk = my video)
OR
( KCl + NH4NO3 => KNO3 + NH4Cl) adding NH4NO3 will make KNO3 which will also preciptate out the same way.
--------------------------------------------
If anyone got any other ideas to make new useful chemicals from thise mixture OR to just seperate them which I still haven't suceed in, please let me
know!
(Also possible, if you know a way to seperate the MnCl2 ,from the KNO3 & NH4Cl OR the MnCl2 from( KClO3 & NaCl , that were formed from the
two reactions above))
[Edited on 7-7-2012 by CrEaTiVePyroScience]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Oxymoron. You have to separate them FROM EACH OTHER. Precipitating the Mn as MnCO3 is by far the easiest and most complete way.
Your chlorate/nitrate routes only substitute one cation for another.
[Edited on 7-7-2012 by blogfast25]
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
Yeah I meant that dont take me on my words dude and it doesn't always have to be easy.
Anyonelse up for some further thinking instead of choosing easiest route?
[Edited on 7-7-2012 by CrEaTiVePyroScience]
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
MnCl2 dehydrates at 58°C maybe thats something?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It hydrolyses/oxidises if you don't do that in HCl gas or inert gas stream. You basically get dirty MnO2. You could leach the KCl from it,
leaving you with the MnO2.
[Edited on 8-7-2012 by blogfast25]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Perchlorate is better than the Chlorate. It is less soluble. But you end up with Sodium Chloride (assuming you added Sodium Perchlorate) mixed with
the Manganese Chloride.
Download the solubilities dictionary from archive.org. It is about 70 mega bytes (big) it will tell you the solubilities of what you have. Fractional
crystallization might work if you pick a solvent that will suit (if it can be had).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dann2  |
Perchlorate is better than the Chlorate. It is less soluble. But you end up with Sodium Chloride (assuming you added Sodium Perchlorate) mixed with
the Manganese Chloride.
Download the solubilities dictionary from archive.org. It is about 70 mega bytes (big) it will tell you the solubilities of what you have. Fractional
crystallization might work if you pick a solvent that will suit (if it can be had).
|
Using an expensive chemical for a simple displacement reaction that creates no real separation? Pointless.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Simply add potassium carbonate solution to your mix. it will make manganese II carbonate and more potassium chloride.
MnCl2 + K2CO3 -) 2KCl + MnCO3
Then heat it at low temperature in open air, exemple in a glass beaker with alcohol lamp for heating. Mix each few minutes the carbonate for full
decomposition/reaction with oxygen.
MnCO3 -) CO2 + MnO
2MnO + O2 -) 2MnO2
You will get manganese dioxide which you can use to make more chlorine gas by adding warm HCl on the dioxide.
MnO2 + 4HCl -) 2H2O + MnCl2 + Cl2
So to make more and more chlorine you will only need potassium carbonate and hydrochloric acid. This process is a variant of the Weldon process. For
the first purification use potassium carbonate to get a filtrate of ''pure'' potassium chloride but after the first reprocessing of the manganese
chloride you could use sodium carbonate which is a lot cheaper than the potassium salt. Sodium carbonate can be made from Sodium bicarbonate by
heating to remove ''excess'' CO2 and H2O.
Hop this helped.
I never asked for this.
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
That's a good answer thank you very much Plante1999 , will make a video of that and give you some credits  . .
If anyonelse would have other ideas or methods I would also love to hear them!
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Oops I forgot to said that after adding the carbonate you need to filter the reaction mixture to get a chloride solution and manganese II carbonate.
Then heat the carbonate in beaker.
[Edited on 8-7-2012 by plante1999]
I never asked for this.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Why heat the Mn carbonate? MnCO3 is soooo much more useful than MnO2!
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
To make more chlorine?
I never asked for this.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
We need LESS chlorine, not more...
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
It depend on what you do... I use chlorine gas a lot and I use MnO2 for it's production... I do not play with manganese chemistry.... Maybe I should
try sometime.
[Edited on 8-7-2012 by plante1999]
I never asked for this.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Why we would need less chlorine, Chlorine is so useful, ''many if not all'' my experiment use chlorine, maybe one day I will try the Deacon
process but at this time I use the Weldon process. The Weldon process is very cheap but not as the Deacon process, but still the weldon process is
better for home chemist. Chlorine production using manganese dioxide start at 30 degree Celsius run smoothly and generally do not have a tendency to
make an uncontrollable reaction as hypochlorite do.
I never asked for this.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes plante, but the purpose here is to recover Mn, not to make chlorine. I've dissolved MnO2 that many times that I'd do a lot to be able to avoid the
Cl2 generating step. I'm pretty sure I'm not the only one who feels this way...
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
The attached table is a useful ready recknor for solubilities.
You can (I guess) add Potassium (or Sodium) Sulphite .....to Hydroxide (in list) in order to precipitate a Manganese compound.
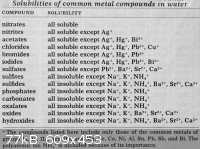
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Assuming you haven't actually done anything to your mix yet, and only want to recover the Mn, dissolve a good amount of NaOH in regular ol' Clorox
bleach and add it to the mix. Instant MnO2-hydrate, no chlorine generated. The leftover supernatant liquid, once filtered, is a mix of KCl, NaOH,
NaCl, and NaOCl.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
Quote: Originally posted by elementcollector1  | | Assuming you haven't actually done anything to your mix yet, and only want to recover the Mn, dissolve a good amount of NaOH in regular ol' Clorox
bleach and add it to the mix. Instant MnO2-hydrate, no chlorine generated. The leftover supernatant liquid, once filtered, is a mix of KCl, NaOH,
NaCl, and NaOCl. |
Then boiling it will form potassium chlorate and more NaCl, chilling precipitates the chlorate, and adding hcl converts the lye to NaCl and everything
can be recovered.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Recrystallization would be needed for anything reasonably pure, but yes.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
| Pages:
1
2 |