| Pages:
1
2
3
4
5
6 |
Maniak
Harmless

Posts: 45
Registered: 26-6-2004
Member Is Offline
Mood: No Mood
|
|
Sure, binder needs right plasticizer. It depends on nature of polymer if it has double bonds or not, if it has more or less polarity, etc. PIB needs
paraffinic oil while naphthenic oil is better for SB. More polar "oils" like phthalates are more useful for polymers like nitrocellulose. However,
exact choice may also depend on mw of the polymer binder.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I would like to have a conversation about plasticizers and binders, unfortunatelly right now I don't know that much about them.
One thing I have wondered about, as I am sure many have, is how well an ETN based plastique stands up against a PETN based plastique. I know that PETN
is much more stable, which is mostly because of a chemical structure which is different than the other common nitric esters. It has that fifth carbon
that ETN doesn't have. I am just interested in the explosive performance right now though. I didn't find data on ETN of course, but I did find some
for MHN which should be close.
The following values were taken from a report by Dave Everest called "The power of high explosives in theory and practice". His references include
several of the "usual sources".
Explosive..........Trauzl Lead Block........Ballistic Mortar
RDX...........................1.64...............................1.50.......
NG.............................1.86...............................1.40......
PETN..........................1.81..............................1.46......
MHN...........................1.68..............................1.36......
TNT was taken as 1.00 for both sets of tests.
Here are some Lead Block results taken from "Explosives 5th edition" by Meyer.
RDX.......1.60
NG.........1.73
PETN......1.74
MHN.......1.70
Its not an exact science but the numbers are fairly close to the first set.
I have noticed that ETN doesn't work as well as PETN in small quantities, like in a cap. I think it was Rosco that mentioned this in another thread as
well. I think it was also said that after the charge size had increased to 1.5-2g or so that ETN performed much better. I could be wrong as I am going
by memory. I should try and find the old posts.
Anyway, if the performance of MHN can be taken as a close approximation of what we can expect from ETN, then ETN's performance is not far behind that
of PETN and RDX. I know this is not news, but I just wanted to do a side by side comparison mostly for my own benefit.
[Edited on 27-4-2012 by Hennig Brand]
|
|
|
hames
Harmless

Posts: 24
Registered: 14-2-2012
Location: oz
Member Is Offline
Mood: as was
|
|
I tried to initiate 200ml of nm+5% monoethanolamine with 2g of etn+200mg of colloidal lead azide and it failed but I'll have to add that it was in a
plastic bottle so there wasn't much confinement to help it along.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Many people get confused on this score: the "motor oil" is actually a synthetic in the patent often quoted. Most commonly a silicone oil with a small
amount of tackifier.
There are generally two ingredients in Deta-sheet: a tackifier and a plasticizer This is not found in SBR as one material does "double duty" and the
"C4" blocks are often not used to mold and shape. Semtex, Deta-Sheet, C6(PA) and others ARE used to mold into door hinges or shaped charges.
Unwrapping a 1/2 lb block of C4 will show a simple, low level plastizer, that easily breaks apart and only forms when very warm. (See "Shaped Charges"
published by the ISEE) That was one reason why poly- ISO-butylene is generally made in situ from polybuelene (like "bird repellant") and a rhinocyte.
The level of particulate material to adhesive is phenomenal: yet this frequently requires rollers or a great deal of "hand manipulation". A 1/2 lbs of
material is generally about the size of a billiard ball, the density is so remarkable.
"Diamond-Charges" from PETN Deta-Sheet are wonderfully effective for the amount of weight [of explosive] used therein. Superficially, it seems that
even though RDX and PETN are similar in powder, speed, etc - RDX is more efficient in larger amounts such as warheads where as PETN is somewhat more
efficient in smaller weights such as base charges in det-caps.
Obviously it wouldn't be appropriate to post actual advertisements for energetics but there OCCASIONALLY are rather in-depth ads with ingredients in
some ISEE publications. There was a very interesting book re: explosive mfg at the turn of the 20th century and there were pictures of the "roller
machines" that did the mixing (titled "100Years of Explosive Manufacture") available through Amazon as a Used book for a few dollars that has a great
deal of information regarding the development of flexible / mold-able energetics.
[Edited on 28-4-2012 by quicksilver]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I just noticed something really interesting when I was looking at the Semtex composition numbers on the Semtex Wikipedia page. I had a hunch and I
plotted the data just to show that my hunch was correct.
Here is the data table taken from the Wikipedia page on Semtex.

Here is a plot of percentage Inerts vs. percentage PETN, for the three different Semtex types, done in excel. Now I don't know about you, but I don't
think I have ever gotten an R^2 value of 1, for any straight line graph, unless of course the data was deliberately set up that way.
Attachment: Percent Inerts vs Percent PETN (23kB)
This file has been downloaded 1185 times
Makes sense really, that Semtex would need more inerts than C4 in order to meet the sensitivity requirements. Simply adjusting the amount of inerts in
proportion to the amount of PETN is quick and easy and obviously gets the desired results (more or less).
[Edited on 28-4-2012 by Hennig Brand]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Ok, I am now convinced that C4 produced in North America and Great Britain uses silicone oil. After reading some patents and a journal on
characterization of C4 explosives, I am convinced.
This does not mean that other oils are entirely unsuitable!
The reasons I see (so far) for using silicone oil are as follows.
1. Much more stable viscosity over a wider temperature range than other oils. This is the same reason why some vehicle shock manufactures switched to
silicone oil.
2. Chemically very stable and unreactive with most other chemicals. This helps ensure longer shelf life even in severe storage conditions.
3. Essentially Non-Toxic. This is important from a handling prospective.
4. Non-Flammable. Having a flammable hydrocarbon mixed with a high explosive seems like added liability.
If people could add, or make changes, to my list I would appreciate it.
Anyway, I have included a couple of figures from the journal I was reading. The journal article is from "Analytical Chemistry", 2010, and is called.
"Characterization of Composition C4 Explosives using Time-of-Flight Secondary Ion Mass Spectrometry and X-ray Photoelectron Spectroscopy"
By
Christine M. Mahoney, Albert J. Fahey, Kristen L. Steffens, and Bruce A. Benner, Jr.
All from the "National Institute of Standards and Technology"
And Richard T. Lareau, from "Transport Security Laboratory"
 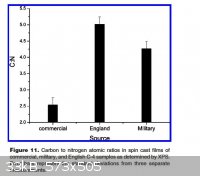
Notice the amount of Si and Oxygen, in the X-Ray Photoelectron Spectroscopy (XPS) results, for English C4. Apparently all three samples, American,
American commercial and English, showed the same general make up, but with variations in proportions.
note:
X-Ray Photoelectron Spectroscopy (XPS), is a quantitative test. There is a good Wikipedia page on it if interested.
Also notice, from the C:N ratios chart, the large variance in the proportion of inerts and RDX between the American, American commercial and English
C4 samples. The English sample had the highest C:N ratio, therefore the highest amount of inerts. The American commercial sample had the lowest C:N
ratio, therefore it has the lowest amount of inerts.
It is obvious from this journal that there are several different types of C4, each with its own composition. It was also clear that all three types of
C4, studied in the journal, are made from the same or very similar components.
[Edited on 29-4-2012 by Hennig Brand]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I had experimented with a British patent some years back and it appeared that the synthetic didn't "weep" from the whole of the mass once it had been
standing for some months.
On another subject the Semtex for export is really very poor quality and very inconsistent in it's composition. The general consensus was that almost
any salvageable energetic was blended into it (that's the "orange" 1/2 lb blocks not the red cylinders). Somewhere I have some scanned material from
the Czech Republic on the government factory and it had some fascinating pictures.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
So, the Semtex specs from the Explosia, a.s. website are most likely "best case scenarios" then, and what is sold usually falls short, unless it is
going to the military. I have read posts by Gerald Hurst saying basically this as well.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Oh, they would put TNT, or just about anything in it and in different proportions. So the only means of getting ANY consistency was the lot number
(which was ink stamped). Many people use the term "Semtex" but there are now several world-wide companies that make a plastique.
One of the most inter4sting stories I had read was about Saudi Arabia and it's design to interrupt it's own oil production of oil if the Kingdom were
overthrown. The major problem was that by using Czech Semtex they had only a true shelf-life of 20 years - then the material would need to be
replaced. Much of this problem had to do with design. The "Petroleum Scorched Earth" concept in oil production is outlined in several books and is one
of the reasons why there is continued work on plastic binders and tackifiers.The ideal material needs to have both, yet last for many decades.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Patent 3173817 issued in 1965 describes compositions which are nearly the same as modern C4. The key difference is that it specifies the use of motor
oil and in brackets it states specifically (SAE 10) in several different places. Is there any type, or weight, of Silicone oil that is specified as
SAE 10?
This patent was issued about the same time that C4 was replacing C3. Is it possible that the early formulation did in fact include nondetergent SAE 10
motor oil and somewhere along the line it was changed to silicone oil?
It is really strange that the proportions used, for the plastic explosives in the patent, match so closely the proportions of modern C4.
|
|
|
Maniak
Harmless

Posts: 45
Registered: 26-6-2004
Member Is Offline
Mood: No Mood
|
|
As it was said here, C4 can not be compared to Semtex in view of mechanical properties, because its desired form is different. C4 is designed to be
used as large blocks where high density is given by rolling. Semtex is designed to be easily hand shaped without changing its explosive properties.
Wiki is wrong about Semtex composition, because octyl phthalate and dibutyl citrate are polar plasticizers and they are not much compatible with SBR.
SBR is used with mineral oil.
Oil used with PIB can definitely be just usual mineral oil. If silicone oil is used in modern composition, it is because of storage or environmental
characteristics.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This is a quote by nitro-genes from another thread on plasticizing explosives:
"It is no coincidence that all plasticizers contain some hydrophilic groups. My theory is that the repellant action of these groups towards the
hydrophobic environment allows for a much better separation of the long chain molecules of the polymer to be plasticized, giving them far better
mechanical properties."
I also found a section a week ago in the book "Handbook of Plasticizers", which also said basically the same thing. I don't own the book and I am
having a hard time finding that exact section in the book again, but I am sure it is there.
Regarding C4 and handling properties; I don't have a reference right now, but I think most of the time you are right about C4 and it is not made to be
molded by hand, etc, but used as is in blocks. However, from memory I seem to remember reading that some C4 is made to be molded and the key
difference is the amount of mixing and kneading which has been done to it. The C4 that is more thoroughly mixed and kneaded has handling properties
more similar to Semtex, I think.
I have included a pdf of chapter 11 out of the "Handbook of Plasticizers", by George Wypych. The chapter is titled "Plasticizers Use and Selection for
Specific Polymers". Section 11.3 is on PIB and section 11.5 is on SBR.
Section 11.5 says it is common to use mineral oils but also dibutyl or dioctyl phthalate to plasticize SBR.
Attachment: Handbook of Plasticizers Ch11.pdf (1.1MB)
This file has been downloaded 83751 times
[Edited on 2-5-2012 by Hennig Brand]
|
|
|
Maniak
Harmless

Posts: 45
Registered: 26-6-2004
Member Is Offline
Mood: No Mood
|
|
Thanks for the piece, it's interesting! But in 11.50 SBR section there is nothing about phthalates.. Maybe you skipped to 11.51 section where they
are, but it is about SBS polymer which is much different. In 11.50 section they mention just some resins and various oils.
About C4 - in my opinion, with 9% of plasticizer-binder you can not reach both high density and moldability in one moment. You can have just one of
them. If you'll knead C4, it will become moldable after some air will be entrapped and therefore density will fall down significantly, followed by
detonation parameters.. In other hand, semtex with around 16% inerts can be shaped and even kneaded without large density decrease.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Oh, sorry about that. I was moving a little too quickly, I guess. As you say, I thought I was still looking at the section on SBR.
You could be right about C4; I don't have the answer right now. I now think though that C4 is not always, 9% inerts, as it is often described in the
more accessible references. I found a modern journal article (2010) which had the results of a detailed quantitative analysis on C4 from different
sources. It was obvious from the results that C4 from different sources can have quite large differences in the amount of inerts. I referenced the
journal article a few posts up.
[Edited on 3-5-2012 by Hennig Brand]
|
|
|
killswitch
Hazard to Others
  
Posts: 209
Registered: 8-7-2011
Location: is a relative concept
Member Is Offline
Mood: No Mood
|
|
I have a question regarding picric acid. Is crystallization from solution the best method for acquiring high density?
A picture of crystals of TNP:

|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Picric acid can be melt-cast for highest density. Pure picric acid melts at ~122.5C. Unlike water most substances, including TNP, increase in density
when they solidify from the liquid state. When picric acid crystals are simply compressed there are many tiny air spaces, which lower the overall
density relative to a solid cast of the explosive. The tiny air pockets make the explosive much easier to initiate though.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I found another interesting journal article. I have included a couple of lines from the abstract below. I am trying to get ahold of the whole article,
but so far no luck. The article is titled,
"Characterization of Semtex by supercritical fluid extraction and off-line GC-ECD and GC-MS"
by
Gregory C. Slack, Harold M. McNair, Lou Wasserzug
Journal of High Resolution Chromatography
Volume 15, Issue 2, pages 102–104, February 1992
"Researchers using electron capture for the detection of explosive vapors currently claim the ability to detect the presence of RDX in Semtex – a
plastic explosive comprising hexahydro-1,3,5-trinitro-1,3,5-triazane (RDX) in a matrix of styrene-butadiene copolymer and hydrocarbon oil."
This would seem to be in agreement with Maniak's assertions about the plasticizers used in Semtex.
Ok, I just found another good article.
"Characterization of Three Types of Semtex (H, 1A, and 10)"
by Stephanie Moore, Michele Schantz, William MacCrehan
Received: November 13, 2009; revised version: December 14, 2009
From Propellants, Explosives, Pyrotechnics
I have included a couple of the results tables from the report.
 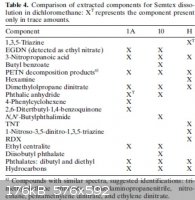
It seems like they do rely heavily on hydrocarbon oils, but some other plasticizers are used as well. It also seems like many different things that
could end up in the mixer.
You know, I don't see any octyl phthalate or dibutyl citrate in either of those lists.
[Edited on 3-5-2012 by Hennig Brand]
|
|
|
Maniak
Harmless

Posts: 45
Registered: 26-6-2004
Member Is Offline
Mood: No Mood
|
|
Sorry, but when I see these analytical articles... I think that it would be better if autors will throw away their costly instrumments (or give it to
us) and then they will just ask manufacturer which materials are used and of which purity. For forensic analysis, manufacturer will probably give them
correct numbers so that they can write some review article 
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This is the abstract, from the article referenced in my last post, which should have been included.
"Abstract
Solid phase microextraction and solvent extraction were used
with GC/MS to determine the vapor and compositional profile
of three samples of Semtex (1A, H, and 10). Semtex is reported
to contain PETN and/or RDX, along with plasticizers, binding
materials, and fuel oil components. In an effort to differentiate
and compare these three variations of Semtex, this report summarizes
the headspace and solvent extraction results for each
material. Components that can be used to differentiate varieties
of Semtex were identified and all three Semtex profiles were distinguished."
I thought I should add the following quote from the same journal as well. It explains why Styrene Butadiene did not show up in the results tables.
"The styrene/
butadiene copolymer took the longest to dissolve in
dichloromethane and once injected, did not provide any
components that eluted from the column. A higher
column temperature (300 C) was applied, but still no
peaks were observed. In a similar fashion, Sudan IV did
not result in any detected peaks."
and also:
"As
previously mentioned, 4-phenylcyclohexene was seen in
the SPME profile of the styrene/butadiene analytical
standard. Since 4-phenylcyclohexene (4-PCH) has no recognized commercial use, it is safe to assume that detection
of 4-PCH at 16.3 min suggests the presence of the
reported styrene/butadiene copolymer in Semtex 1A."
I probably should have included tables 1 & 2 which have the results of testing the standards. Tables 1 & 2 show the results gotten from
testing pure samples of known/suspected Semtex components, obtained from chemical supply houses. Here are tables 1 & 2, from the journal
referenced in my last post.
 
[Edited on 6-5-2012 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
killswitch
Hazard to Others
  
Posts: 209
Registered: 8-7-2011
Location: is a relative concept
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | Picric acid can be melt-cast for highest density. Pure picric acid melts at ~122.5C. Unlike water most substances, including TNP, increase in density
when they solidify from the liquid state. When picric acid crystals are simply compressed there are many tiny air spaces, which lower the overall
density relative to a solid cast of the explosive. The tiny air pockets make the explosive much easier to initiate though.
|
I tried that... While parts of it melted, chunks of it seemed to be vaporizing and condensing on the upturned beaker I'd placed over the container. It
was transparent, though. Was it actually water?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I don't have any personal experience with casting Picric Acid. I do know that it was done a lot, especially in WW1. For safety an oil bath should be
used and no open flame (use a hot plate). I would make sure the Picric Acid is as pure as possible; some contaminants can increase sensitivity and add
to the danger(metallic impurities especially). Keep in mind even pure picric acid is much more sensitive in the hot, molten state. Picric Acid is
often mixed with other lower melting point explosives, making a mixture with a lower melting point than pure picric acid.
The vaporizing chunks could maybe be vaporizing or subliming dinitrophenol. Taken from Wikipedia:
"2,4-Dinitrophenol is a yellow, crystalline solid that has a sweet, musty odor. It sublimes when carefully heated and is volatile with steam, boiling
point 113 C....Picric Acid boiling point > 300 C, explodes."
Maybe all one has to do to remove the DNP from their TNP sample is to distill it off.
I think I am starting to see why I always seemed to loose a little weight during the times I was experimenting with Picric Acid. There are times
during a TNP synthesis when a person could be exposed to more DNP than you might think. When recrystallizing TNP I noticed anywhere the vapor would
condense that there would be a bit of yellow stain. DNP being so much more volatile than TNP, if present, would have been the major component of the
dye in this "yellow steam" (I think).
I hope I have helped some. I have made TNP quite a few times, but truthfully I have never cast it.
[Edited on 7-5-2012 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Shellite in England or Sprengstoffe 88 in Germany, used during WWII to fill large caliber shells for battle ships was TNP plus few percent of
nitrophenole, added most likely to decrease melting point. I made experiments with melting TNP. Sulfur bath works well. But I used some shit instead
of good primer- Ag2C2 and was unable to detonate TNP. (Complex salt Ag2C2.AgNO3 had to be used instead). It was not very simple to fill shells with
TNP. These shells had to be covered inside with pure Sn without Pb and cooling had to be done under hi pressure (compressed CO2), otherwise there was
porous in solid TNP. Finally I stopped experiments with TNP and converted it to its ammonium salt. Nice compound, insensitive (less than TNT) and can
be mixed with AN.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
You initiated cast TNP with double salts? From my experiences with picric acid, at least in caps, I am surprised double salts (in reasonable amounts)
even detonate compressed crystalline TNP. The ammonium salt (explosive D or ammonium picrate) is quite a bit less powerful than picric acid and is
very insensitive. The only advantage of explosive D (militarily) was that it was so insensitive that it could be driven through the side of a steel
target without detonating and then be detonated on the inside. I find for normal/non-military uses even pure picric acid is more insensitive than I
would like most times.
So you mix ammonium picrate with AN, kind of like they do with TNT? I have read the section in Fordham several times regarding TNT powder. IIRC it is
normally around 10% TNT / 90% AN and it is certainly cap sensitive. What proportions did you use when you mixed your ammonium picrate with AN? How
sensitive to initiation was it?
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
You misunderstood me a little. I did not cast mixtures of TNP with its salts. I made one experiment with pure TNP and did not repeat it. I used later
mixtures of ammonium picrate with AN and Al. I do not remember proportions, most likely they are of no great importance. Make your own experiments. I
used TATP to initiate aforementioned mixture and according my own experience it is powerful primer and not as dangerous as many people think (HCl must
be used as catalyst, not H2SO4 !). I read about solid propellant, based on ammonium picrate: 45% of AP, 45 % of NaNO3 and 10 % of some binder (epoxy?)
but I've never tested it. Another advantage of AP is that it is not an acid. Kalium picrate is an interesting salt too. It is more sensitive than pure
TNP but does need a primer- it explodes, been ignited in confinement.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I don't think I know much about ammonium picrate/AN/Al, but the aluminum would probably be most responsible for sensitizing the mix.
You find TATP to be a powerful primary; what did you compare it to? I found TATP was fine for initiating dynamites and some other sensitive
secondaries. I found it was next to useless for setting off TNP in a compound cap. TATP is very powerful when compared to something like potassium
picrate though.
I have experimented with potassium picrate a few times and see that it may have some uses in a flash igniter or something. Potassium picrate is really
very weak, I think one of the lead salts would be a much better option. If you can get the process down and have good reactants, basic lead picrate is
really quite impressive (much better than the potassium salt).
TATP is dangerous like all primaries are dangerous, but worse than most primaries used by hobbyists it is considered very unpredictable. You might get
away with using it a certain way 100 times and then at 101 you lose two fingers. I have never had any problems with it, but I consider myself
fortunate.
I am not saying I would never experiment or use TATP, but I would be much more cautious with it than most of the other explosives I experiment with.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
2
3
4
5
6 |