| Pages:
1
2
3 |
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bot0nist  | I don't believe the OP asked anything about ingestion of tryptamine or its methylated variants. I do hope that the original poster has read these
three threads on decarboxylation of tryptophan right here on SciMad. I also saw some patents on the subject IIRC.
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=8574#pid97905">L-tryptophan decarboxylation</a>
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=2255#pid23507">decarboxylation of tryptophan</a>
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=3544#pid39231">tryptophan decarboxylation</a>
|
I feel left out   ( ( ) )
http://www.sciencemadness.org/talk/viewthread.php?tid=14147#...
@GreenD: Spearmint/caraway oil decarboxylation works great! Also the student workup is a godsend for people without a decent vacuum pump. Try
fractionation of the carvone, degassed solvent and inert atmosphere.
| Quote: | | On a similar note, what can be done with phenylethylamine? You can buy tons of pure PEA hydrochloride from some supplement website. Nothing naughty of
course. |
Oxidize to benzyl cyanide, one pot Gringard/NaBH4 and reduction to the isopropylamine. Well, some people would consider that naughty. 
There is an Iranian article on oxidation of amines to cyanides with TCCA. As with anything out of Iran/India/China to be taken with a grain of salt.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
I found the paper in the References section "Direct Oxidative Conversion of Alcohols, Amines, Aldehydes, and Benzyl Halides into the Corresponding
Nitriles with Trichloroisocyanuric Acid in Aqueous Ammonia"
Its incredible, almost too good to be true. TCCA can oxidize whatever you put into it and turn it into a nitrile at the same time. You could make tons
of benzyl cyanide that way. OrgSyn has a good purification procedure
Maybe tryptamine could be turned into indoleacetic acid. Make some plant hormones
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
that would be quite the overkill to get iaa...
the wikipedia of IAA has probably the simplest reaction 
|
|
|
antibody
Harmless

Posts: 38
Registered: 4-2-2011
Member Is Offline
Mood: No Mood
|
|
Would hyrdoboration of the indole double bond not be a competing rxn using Zn(BH4)2 for alkylation or amide reduction?
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Quote: Originally posted by ParanoidAndroid  | I have a large amount of pure tryptophan and recently came across an exceedingly simple method of making it into tryptamine: basically just reflux it
a mixture of xylene and acetone until CO2 stops evolving. A spearmint oil catalyst can be used to speed the reaction but adds a purification step
afterward and reduces yield.
My question is: what can I do with the resulting tryptamine? There are a whole slew of interesting substances that are related to tryptamine, but there's very little information on synthesizing those substances using tryptamine as a starting
material. Are there any easy ways to do this?
Of particular interest to me are AMT (an MAOI), serotonin (neurotransmitter), and DMT (which is an interesting precursor to other syntheses). The
molecular changes aren't complicated; there just isn't a lot of experimental info out there. |
Quote: Originally posted by Bot0nist  | Decarboxylate and post a write up with the procedure, yield, and product identification ( melting point, solubility's, etc) before even worrying about
methylation.
[Edited on 22-12-2011 by Bot0nist] |
So, any luck with that decarboxylation android. Waiting for that write up...
[Edited on 14-2-2012 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
The "exceedingly simple method" ParanoidAndroid "came across" and failed to give a reference to (why is it that Newbies always refuse to give
references?) is probably fiction. Xylene boils at ~140°C - distinctly lower than the solvents used in verified methods. It'll probably take a long
time. But I have not tried it.
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Quote: Originally posted by turd  |
The "exceedingly simple method" ParanoidAndroid "came across" and failed to give a reference to (why is it that Newbies always refuse to give
references?) is probably fiction. Xylene boils at ~140°C - distinctly lower than the solvents used in verified methods. It'll probably take a long
time. But I have not tried it. |
It works, but at 40 hours+ at heavy reflux with cyclohexanone as ketone, though I doubt acetone would really be the catalyst of choice due to the way
to low bp.
Acetophenone as solvent and catalyst does it nice and clean in less than 2 hours, though I never came up with a good separation of the stuff, so it
went down the drain after a month in the fridge...
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Quote: Originally posted by bahamuth  |
Acetophenone as solvent and catalyst does it nice and clean in less than 2 hours, though I never came up with a good separation of the stuff, so it
went down the drain after a month in the fridge... |
distill it...?
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Quote: Originally posted by GreenD  | Quote: Originally posted by bahamuth  |
Acetophenone as solvent and catalyst does it nice and clean in less than 2 hours, though I never came up with a good separation of the stuff, so it
went down the drain after a month in the fridge... |
distill it...? |
At the time I didn't have access to a short path, or a vacuum source below 1 mbar, only one around 14 mbar.
Perhaps the tryptamine would survive the harsh conditions with the acetophenone passing over first, but doubt it.
On a later time I distilled N,N-dipropyltryptamine through a short path with at very low pressure (0.001 - 0.1 mbar) and it worked perfectly, but got
greedy and the final product, an oil, got a slight discoloration though before the greedy part the oil was perfectly colorless.
If I were to repeat decarboxylation of tryptophan I would atleast test the intrigueing precipitation of the tryptamine with CO2.
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
What would be the source of CO2 - just some dry ice bubbled through a tube?
Nicodem (I think) suggested this; saturate ethanol with fumaric acid. Pour ethanolic solution into acetophenone - decant to get wanted salt?
|
|
|
cgnmxdq9
Harmless

Posts: 1
Registered: 20-4-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by GreenD  | What would be the source of CO2 - just some dry ice bubbled through a tube?
Nicodem (I think) suggested this; saturate ethanol with fumaric acid. Pour ethanolic solution into acetophenone - decant to get wanted salt?
|
I did something similar to this to wash the tryptamine (after the allowing the aceophenone to evaporate) using acetone instead.
At the time of its synthesis I did not have anhydrous/reagent grade acetone on hand so I just kind of let it sit around until I did.
Now I have a bunch of tryptamine fumarate lying around that I don't know what to do with.
aMT seems a both a legal (in most places) and interesting option to explore though its synthesis from tryptamine looks like it would be troublesome.
There are also probably ways to get it to do interesting things in vivo but reading papers of tryptamine's neurotoxicity turned me off to this idea.
All in all? Meh.
[Edited on 20-4-2012 by cgnmxdq9]
|
|
|
querjek
Hazard to Self
 
Posts: 76
Registered: 26-8-2008
Member Is Offline
Mood: No Mood
|
|
Would steric hindrance stop the amine from overreacting with isopropyl bromide? ethyl bromide?
it's all about chemistry.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
ethyl bromide gives the tertiary compound as the main product.
isopropyl bromide may only give the secondary amine as the major product. The di-isopropyl amine has been made though.
N,N-EthylIsopropyl amine is reportedly active.
ʃ Ψ*Ψ
Keepin' it real.
Check out my new collaborated site: MNMLimpact.com
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
whenever i have posted questions of this kind i have been attacked, check out my thread http://www.sciencemadness.org/talk/viewthread.php?tid=19194#... , i dont know whether it works though  , im not going to risk getting ass raped for 7 years , im not going to risk getting ass raped for 7 years
http://en.wikipedia.org/wiki/Tryptophol , it mentions "splitting off carbon dioxide and re-placing the amino group with hydroxyl"
i assume its a two stepped enzyme based reaction
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Thanks for this. I know him and this is certainly true.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Some people only like to blow shit up on here. Others enjoy introverted analysis. Sometimes the two don't mix. A perfect example is Rosco Bodine.
ʃ Ψ*Ψ
Keepin' it real.
Check out my new collaborated site: MNMLimpact.com
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
im interested in all parts of chemistry, including blowing things up, i made about 500mg of purified manganese heptoxide, i cannot stress how
dangerous and awesome that compound is, i dropped an ant in it :3,
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Diethyltryptamine?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
this
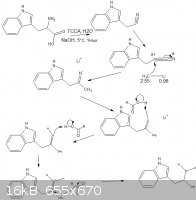
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Please stay in the beginnings forum with these pipe dreams. Generally: Don't cross post.
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
Try this
http://www.google.com/patents?id=8QwgAAAAEBAJ&pg=PA6&...
|
|
|
Noshtuba
Harmless

Posts: 6
Registered: 16-5-2015
Member Is Offline
Mood: No Mood
|
|
I ve tried it using sodium triacetoxyborohydride(not generated in situ). didnt work. vry low yield. i would like to try the zinc borohydride method.
but the solvent thats documented is dcm. im afraid tryptamine react with it before it gets methylated? i dont know.
Tryptamine is easily made from tryptophan. i've done it using it acetophenone. the purification is where most people get stuck. Simply extract with
acetic acid, wash with dcm, basify, put the whole thing in the freezer overnight then put in the fridge. dirty crystals of T appear out of solution.
filter under vacuum. i ve found that ammonia is an excellent recrystallization solvent. Use room temp ammonia to dissolve the crystals, filter the
brown impurities, put the solution in the freezer. vacuum filter. gets the job done without vacuum distillation.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
https://www.erowid.org/archive/rhodium/chemistry/psilocin_sy...
Seems to be an efficient existing synthesis utilizing NaBH4/NaCN. I don't like using NaCN myself, but some times you hafta do things you don't like.
This pathway is reputed to work, and there just ain't very many "easy" pathways available.
In the synthesis of Dimethyl Tryptamines, there always seems to be some unpleasant obstacle.
|
|
|
Noshtuba
Harmless

Posts: 6
Registered: 16-5-2015
Member Is Offline
Mood: No Mood
|
|
You d still need the sodium cyanoborohydride though. thats the only pathway i know of which is guaranteed to work. shame it's hard to get
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. I've heard that the reagent isn't easy to come by. And, making it involves using HCN. Not fun.
|
|
|
| Pages:
1
2
3 |