niertap
Hazard to Self
 
Posts: 76
Registered: 5-8-2011
Member Is Offline
Mood: hyper-conjucated
|
|
Ellement Collecting: finding/synthesizing
A discussion of synthesizing and finding the elements.
On my list of current ideas are reducing the following to their elemental form. Ba(NO3)2, Sr(NO3)2, ammonium molybdenate, and ammonium metavandate.
What all has everyone tried or collected?
Ignorance is bliss
Outliers in life are modeled by chemical kinetics
|
|
|
Ramblesthegoat
Harmless

Posts: 14
Registered: 18-2-2012
Location: Upstate NY
Member Is Offline
Mood: No Mood
|
|
Well, you can get Sr and Ba through electrolysis, but the other chemicals you mentioned might be harder. Just convert the nitrates into halides, melt
down the chemicals and pass a strong current through them with non reactive electrodes ,most people use carbon (graphite).
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Expect problems with carbon electrodes. They have a high resistance, so you might need to pump more current through them than the cell's efficiency
level. A great how-to on electrolysis (of sorts) can be found here: http://www.youtube.com/watch?v=i9xS9t-KMpc
I wrote an 80+-page book on collecting elements, so if you have questions, just ask.
What are your plans for the ammonium metavanadate, and how did you obtain it?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
You can make vanadium using ammonium metavandate by first heating it to about 200 degrees celsius, (http://en.wikipedia.org/wiki/Vanadium(V)_oxide)
forming V2O5, then make the metal through thermite with aluminum/magnesium powder.
Molybdenum can be made by acidifying the ammonium molybdenate, extracting the oxide formed, then making the metal through thermite (again).
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Ammonium metavanadate IIRC is very expensive, and V2O5 is actually quite cheap from pottery suppliers and I think I've seen it a few times on eBay -
so if I were you, I'd start there and conserve your supply of the former.
Don't forget displacement reactions - you'll have to be pretty selective for which elements to extract using this method, but it can work a treat for
many of them. Reductions are also a possibility if you have the right gear, especially for some of the more reactive metals such as potassium, perhaps
sodium, maybe even some of the alkali earths as well, as was discussed for a long time on this board and the German equivalent. NurdRage made a video
of it on his YouTube channel, so for a visual demo check him out.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
1. When dealing with elements we say "isolating" rather then "synthesizing" unless you are either God or work in the radiochemistry.
2. Thermite is not the answer as it may seem; V will be very impure (about 50% IIRC) while Mo can be recovered from lightbulbs - do your homework.
Hint: it's not the filament.
Sr and Ba can be reasonably obtained with Ca reduction, however some special aparatuys must be built. Ba is tough to get purer then 90%. Brauer has
good descriptions of the processes.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
With regards to the molybdenum, to be slightly more helpful than a_bab, molybdenum is used to make support wires for the filaments.
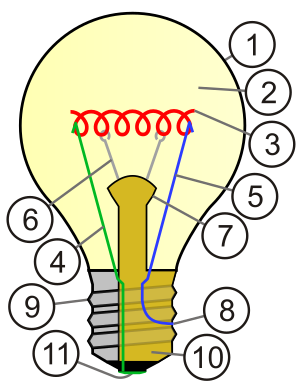
In this diagram, I think the Mo is in position 6.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Hexavalent, where is that diagram from? I'm interested to know what all the other numbers refer to. Lightbulbs are a surprisingly rich source for the
element collector - I've gotten 3 from them so far: argon (2), tungsten (3), and molybdenum (6).
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Not counting the special krypton, xenon, and mercury types.
Furthermore, I prefer to take barium out of a vacuum tube, they use it as the 'getter'.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
thethule
Harmless

Posts: 9
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
Can i ask, where one could go about having a look/buying this book?
Cheers
Marc
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i used to have 74 of the 90+ elements in little jars and i am currently working on a phosphorus (white and red ) i know about the big topic!
but i be glad to help you as well as reading that 80 page ! sounds very interesting!
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
mrhomescientist, I think the image was from the Wikipedia page for Incadenscent light bulbs . . .here is the full list of what the numbers mean;

1. Outline of Glass bulb
2. Low pressure inert gas (argon, nitrogen, krypton, xenon)
3. Tungsten filament
4. Contact wire (goes out of stem)
5. Contact wire (goes into stem)
6. Support wires (one end embedded in stem; conduct no current)
7. Stem (glass mount)
8. Contact wire (goes out of stem)
9. Cap (sleeve)
10. Insulation (vitrite)
11. Electrical contact
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Lamp autopsy:
http://www.teralab.co.uk/Glass_Blowing/Lamp_Autopsy/Lamp_Aut...
|
|
|
pedrovecchio
Harmless

Posts: 38
Registered: 12-3-2012
Member Is Offline
Mood: No Mood
|
|
Here is a paper that shows simple methods of making oxygen and chlorine from bleaching powder. The latter is catalytically decomposed to oxygen by
cobalt oxide, and also oxidizes hydrochloric acid to chlorine. Clear instructions are given and simple equipment is used.
Attachment: krauskopf1935.pdf (1.5MB)
This file has been downloaded 888 times
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
I don't have an element collection but theres a few elements I want to obtain because they are so mysterious to me. One is yttrium. I have never heard
anything about this element. Another one is astatine. Its a mysterious halogen that I've never heard anything about. I'm fascinated by promethium
because all the pictures I've seen show it as a glowing green substance but I stay away from radioactive substances for obvious reasons. I'm guessing
they can be safely stored in the home lab in the right container though.
|
|
|
Funkerman23
Hazard to Others
  
Posts: 416
Registered: 4-1-2012
Location: Dixie
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | Not counting the special krypton, xenon, and mercury types.
Furthermore, I prefer to take barium out of a vacuum tube, they use it as the 'getter'. |
Don't use any of the
early tubes..look for ones made during and after the 60's for Barium. Kindly avoid "harvesting" certain tubes though: 6T5, 1629, 6E5..Eye tubes
mostly.Those are not often made anymore and I only know of 5 factories making ANY eye tubes at all...and those have a Magnesium getter more often than
not. Loktal Tubes are also not so good for Barium as far as I know. Plenty of Molybdenum in them though: a lot of the grid wires where made from it.
if you want to scrape off the white emitter you might even get Strontium from old tubes.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Quote: Originally posted by mycotheologist  | | One is yttrium. I have never heard anything about this element. Another one is astatine. Its a mysterious halogen that I've never heard anything
about. I'm fascinated by promethium |
Promethium and Technetium are very rare elements because they do not have any stable isotopes some of them are long lived and can be stored safely
(like uranium) some other isotopes are too radioactive and glow (like radium)
Technetium is often used as a Xray source in portable radiography set up.
Astatine has been isolated in the mg quantity but never really found any industrial or medical applications .
Yttrium can be found in a few minerals some old TV tube contained the phosphate for the color red. Y is not as rare as the others though
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
"Astatine has been isolated in the mg quantity " - you meant in micrograms (μg) amounts.
Besides it is made rather then isolated. One of the little cases where an element is created.
[Edited on 16-4-2012 by a_bab]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes thats correct i meant ug (cant find the micro sign...)
by neutron capture of Bi209 we get At211 with a half life of 7.2 hours..
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by neptunium  | yes thats correct i meant ug (cant find the micro sign...)
by neutron capture of Bi209 we get At211 with a half life of 7.2 hours..
|
To make the symbol "μ", type "μ" (that's ampersand-"m"-"u"-semicolon) in the editor. If
you ever want to see how somebody wrote something, just start to reply to the post and you'll see the raw markup in the editor. (Remember not to reply
if that's all you're doing.)
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I'm back! 
@Funkerman23, I had no idea. I assumed that all tubes in production were barium tubes.
@thethule and neptunium: Attached, and hopefully readable.
Attachment: Getting the Elements.docx (92kB)
This file has been downloaded 1891 times
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|