Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
lauryl alcohol synthesis
I am attempting to make lauryl alcohol using a Bouveault-Blanc reaction per OrgSyn. The workup for this is giving me a lot of trouble. If anyone
here has run this synthesis before, please let me know as I sure could use some help.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2758
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
I think the challenge of that is that the ethanol will react with the sodium to form sodium ethoxide, which will transesterify the esters to biodiesel
as a competing reaction. There is some trick to doing that reaction in such a manner than you prevent or slow that step.
An alternate route is shown below in a JOC paper. It sound much better. One suggetion might be to actually use the methyl esters to start (aka
biodieselm B100) as that will be much easier to work with, and already clean and pure if you buy it commercially. It is also cheap at $4 / gallon.
http://signachem.com/wp-content/themes/signa/pdf/technical-d...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks Dr Bob for your comments and the JOC paper. I note that that the paper's authors got a 65-75% yield making lauryl alcohol (no.8 of Table 2)
using the classic Bouveault-Blanc procedure. I would be quite happy with that yield.
I think it likely that my problem stems from impure ethyl laurate, which I made myself from coconut oil. I suspected this before but really never
checked it for "free fatty acids (FFA)". This is something the biodiesel makers are concerned with as shown in this excerpt from the "Jouney to
Forever" site:
"High FFA levels
Most people find their used cooking oil generally gives a titration of 2-3 ml, but some used oils can have much higher FFA levels than this --
we've seen horrific titration levels of 9.6 ml. "Horrific" because FFAs are not good for you -- it's a bad idea to eat food from a restaurant that
does that to their cooking oil. Another biodieseler reported restaurant oil with titration levels of 16 ml -- black stuff with the consistency of sump
oil."
I just titrated my ethyl laurate using 0.025N NaOH as do the biodiesel folks. My value was 3.8mL. This is much more than I expected. I also added 4
drops of 4M NaOH to 2 mL of my ethyl laurate and soap immediately formed and settled to the bottom.
So, to verify my Bouveault-Blanc technigue I need to obtain some high grade ethyl laurate. Perhaps B100 biodiesel would be a good test also, but I
realize that it would likely be a methyl ester of unknown fatty acids, ie, not necessarily a laurate.
A comment on the Bouveault-Blanc reaction: I have done it twice now at 1/5 scale of OrgSyn using a 1000mL RBF. Although it gets quite hot, at no time
did I feel that it was unsafe. I did use an ice-pack to cool it off somewhat.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is a report on my 3rd attempt to make lauryl alcohol via a 1/5 scale version of the OrgSyn procedure, which uses a Bouveault-Blanc
reaction. Although the yield was a disappointing 40.3%, at least some crude alcohol was obtained.
The Bouveault-Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. In my
case:
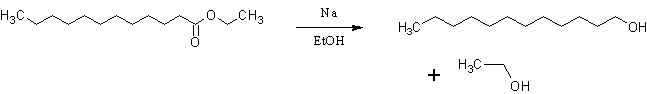
This time I made sure my reagents were good. I dried my ethanol by refluxing distilled spirits with Ca(OH)2 (slaked lime), distilling it, then
storing it over 20 volume % 3A mole sieves. It tested negative for water with both the CuSO4 and CaC2 tests.
My ethyl laurate was purchased to insure purity, especially from free fatty acids.
Another change I made was to use a regular 200mm reflux condenser rather than the much longer one I had made from a 3/4" copper water pipe.
Furthermore, I attached a CaCl2 drying tube to the outlet of the condenser. See setup below:

1. Bouveault-Blanc reactor
To the 1000mL 3-neck RBF was added 40mL of anhydrous toluene (distilled and stored over 3A mole sieves) and 14g of Na in small chunks. The oil bath
was set at 120C and the toluene heated until the sodium had melted. At this time the stirrer was turned on to disperse the Na into droplets. The oil
bath was then removed, and 22.8g of ethyl laurate in 30 mL of ethanol, and then 100 mL of ethanol, were added as rapidly as possible through the
pressure-equalizing funnel, all the while stirring vigorously. Significant heat was generated nearly overwhelming the condenser. An ice-pack placed
against the RBF was used to keep the temperature under control. The following picture shows the products following the reaction:

2. lauryl alcohol
The waxy looking material on the surface is, I believe, lauryl alcohol. The large floating object is a piece of unreacted sodium. Per procedure I
heated this product for about another hour on a steam bath just to get that piece of sodium dissolved.
The product was then steam distilled to remove toluene and unconsumed ethanol. I distilled until I could no longer smell toluene in the distillate.

3. steam distillation
The product mix had by now turned brown. It was poured into a separatory funnel while still hot and immediately washed three times with 40mL of near
boiling water. The wash water from the first 2 washes was watery and cleanly separated from the dark brown product:

4. product after 2 washes
The wash water from the 3rd wash was totally different and appeared to be mostly soap, ie, sodium laurate. This can be seen in the picture below
where the 1st two wash waters are in the large beaker and that from the 3rd wash is in the small beaker:

5. lauryl alcohol after 3 washes
The dark brown washed product was then washed with 25mL of ether. The wash water from the 1st two washes was also washed with 25mL of ether. The
wash water (soap) from the 3rd wash was discarded.
The etherates were combined and centrifuged, retaining only the clear supernates.
This clarified etherate was then washed succesively with water, Na2CO3 solution, and water again, and then dried over anhydrous MgSO4. The 40mLs of
dried etherate was then placed in a small beaker and the ether allowed to evaporate, leaving about 9mL (7.5g) of crude lauryl alcohol:

6. crude lauryl alcohol
The product is a waxy solid at room temperature but melts in the beaker when hand held. Melting point for lauryl alcohol is 24C. The final step of
purification is to distill this product under reduced pressure. I may wait for more product before doing that.
Discussion
This third attempt proceeded way better than the previous attempts. There was virtually no foaming and I really saw no soap until the massive
formation in the 3rd wash.
I'm thinking that instead of steam distilling for an hour to dissolve that big chunk of Na I should have just manually removed it. Eliminating the
long steam distillation might have significantly reduced the amount of carmelization indicated by the dark brown color.
Also, the one 200mm condenser did not have sufficient capacity. Next time I would link my 200mm Hempel column in series with it as it also has a
water jacket.
As usual, questions, comments, and suggestions are welcomed.
[Edited on 25-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Magpie  |
I dried my ethanol by refluxing distilled spirits with Ca(OH)2 (slaked lime), distilling it, then storing it over 20 volume % 3A mole sieves. It
tested negative for water with both the CuSO4 and CaC2 tests.
|
The above statement is in error. What I meant to say was: ...CaO (unslaked lime)..., instead of Ca(OH)2 (slaked lime). This was probably obvious to
those "skilled in the art." 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
francis
Hazard to Self
 
Posts: 72
Registered: 1-4-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
What are the CuSO4 and CaCl2 tests?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
1. Add a small amount (few mg) of anhydrous CuSO4, a white powder, to a mL of the ethanol. If the powder turns blue (forming the hydrate) there is
water in the ethanol. If it doesn't, the alcohol is anhydrous.
2. Add a small amount of CaC2 to a mL of the ethanol. If water is present you will see a stream of tiny bubbles, which is acetylene. If you don't
see any bubbles the alcohol is anhydrous.
Ref: Unitized Experiments in Organic Chemistry, by Brewster et al, 1960.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
madelectrolyzer
Harmless

Posts: 3
Registered: 4-4-2012
Member Is Offline
Mood: No Mood
|
|
Elsewhere (Organikum, 21. edition, german language organic chemistry cook book)
isopropanol (easy to procure in anhydrous form) is suggested instead of ethanol.
(this work can be downloaded in entirety from usenet)
I ran the original through google translate and it's decent enough with a few edits.
Lauryl alcohol is one of the tested preparations with a suggested yield of 80%.
General Procedure for the Bouveault Blanc reduction of esters and nitriles (Table 7.264)
Attention! When dealing with concentrated sodium and lye wear safety goggles!
Great care must be taken in decomposing the reaction mixture. The water may only
be added if all sodium residues are gone.
All parts of the apparatus and reagents must be completely dry. The alcohol is
preferably dried after the magnesium method), the xylene over sodium and the
Ester or the nitrile by vacuum distillation.
In a 2-liter three-necked flask equipped with Hershberg stirrer (see Fig A.6g), intensive cooler with
Calcium chloride tube and dropping funnel 4.5 mol of sodium and a pinch of stearic
acid (as emulsifier) are heated under 800 ml (1000 ml with 0.5 mol of dicarboxylic acid esters)
Xylene to melting. Then the heating is removed and the stirrer set in motion.
The mixture is stirred very vigorously until all the sodium forms a fine gray-dispersion
and, without further stirring, left to cool below the melting temperature of sodium.
Now, again with vigorous stirring, a mixture of l mol (or 0.5 mol of dicarboxylic
esters) carboxylic acid ester or nitrile and 3.5 mol of isopropyl alcohol from the dropping funnel
are added as quickly as the capacity of the cooler allows.
The mixture is stirred for another 15 to 20 minutes, and then as much methanol
as needed to decompose the leftover sodium is added. Finally, carefully mixed with 800 ml of water.
After cooling, the phases are separated and the aqueous phase is extracted with ether
extract (be careful because of emulsion formation) with diols 5 days in the perforator. organic
phase and ether extract are combined, dried with sodium sulfate and distilled with a 40 cm
Vigreux column.
Be always very careful with elementary sodium. YMMV.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by madelectrolyzer  | Elsewhere (Organikum, 21. edition, german language organic chemistry cook book)
isopropanol (easy to procure in anhydrous form) is suggested instead of ethanol.
(this work can be downloaded in entirety from usenet)
I ran the original through google translate and it's decent enough with a few edits.
Lauryl alcohol is one of the tested preparations with a suggested yield of 80%.
|
Thank you for posting this! When was it published? Who were the authors?
I just acquired the 1922 procedure of Roger Adams and CS Marvel as published in the University of Illinois bulletin Organic Chemical Reactions
IV. I've compared it to the 1930 procedure in OrgSyn by SG Ford and CS Marvel. They are similar but I do like the OrgSyn
procedure better based on my reading of the two only.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
As posted above, earlier this year I made several attempts at making lauryl alcohol (n-dodecanol) using the Bouveault-Blanc synthesis. I found this
method not only fascinating, but very difficult. In one of my attempts I was able to get a 40% yield of 9mL (7.5g). This morning, very much wanting
to do some chemistry, I decided to purify my crude product. So, I poured it (mp = 24C) into a 25mL RBF and vacuum distilled it. I didn't make any
provisions for bumping, which was a mistake. Using an air condenser the distillate came over at 150-155C at P = 15mmHg. OrgSyn says to expect it to
boil at 143-146C at 18mmHg. Now if I still have some chlorosulfonic acid I will make a little detergent.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
3.6 mL of the purified lauryl alcohol was reacted with 1.05mL chlorosulfonic acid in glacial acetic acid using a 3X procedure found in "Introduction
to Organic Laboratory Techniques, " by Pavia et al. The product was neutralized with an excess of Na2CO3, and then extracted with n-butanol. After
drying for 3 days in the sun there was still a smell of butanol so drying was continued for 3 hrs in a 123C drying oven. Yield was 4.4g of the
product sodium lauryl sulfate for a %yield of 94.8%. Sudsing ability was equal to that of Tide detergent. The product is shown below:

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
That looks like some squeaky clean product there, Magpie! Congratulations on your synthesis. An excellent yield of final product; any pictures of the
sulfonation? Any ideas for other uses of lauryl alcohol? And further intentions for the SLS?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by DJF90  | | That looks like some squeaky clean product there, Magpie! Congratulations on your synthesis. An excellent yield of final product; any pictures of the
sulfonation? Any ideas for other uses of lauryl alcohol? And further intentions for the SLS? |
Yes, I was pleased with the synthesis as the lauryl alcohol and the chlorosulfonic acid were laboriously homemade. When I had done this synthesis
years ago in organic class using 1X scale the yield was 68%.
I didn't take any pictures of the sulfonation. It was interesting, however, as the mix went quickly from cloudy to clear back to cloudy, with an
immediate gas evolution. I'm not sure what that gas was - HCl maybe? The mix did not get very hot. 15mL water was then added, as well as the
Na2CO3. The product mix was then extracted twice with 9mL of n-butanol. I have no further plans for the SLS at this time.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2758
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Nice work Magpie. Chlorosulfonic acid is not the easiest thing to make or work with. And getting clean colorless product is hard in most
reactions.
You can wash your hair with it. Most shampoos use it along with several other detergents and some long chain alcohols.
You should make some benzyl dialkyl ammonium chlorides/sulfates next. Otherwise know as Lysol bathroom cleaner, as well as in many disinfectants.
(They are also used in some hair conditioners to go with your shampoo.) I worked a summer job years ago at a R & D lab that made many variations
on those, and they had made hundreds of variations trying to find better ones, few worked any better than the R = C12-C14 ones that are still widely
used. Some of the Triton detergents are what is in the "Clean Shower" type products that clean your shower if you actually use them. They add some
Ammonium ETDA in order to help chelate metals (I'm guessing mostly Ca and Mg) and isopropanol to help with organic residues. I made some up a few
years back.
|
|
|