| Pages:
1
2 |
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
wet aminative reduction
The aminative reduction is important to make amines from carbonyl compounds like ketones and aldehydes.
I searched a lot to find a good method and there are many from using Al/Hg to NaCNBH4 even those using NaBH4 on anhydrous condition, and others, and
others.
The problem is
1- I don’t like to deal with HCN or CH3NH2 gas
2- I don´t like to use mercury and the Al/Hg reaction gives to me poor yields and it is, well, a mess.
So, I am stuck to do the aminative reduction using NaBH4 and not on anhydrous condition since I use methylamine 40% solution.
“There is a relatively rapid formation of the imine and the imine is reduced relatively rapidly. There's no reduction of the ketone to the secundary
alcohol, as one might expect. In similar reactions, the water that is produced during the forming of the imine (Schiff Base) is removed (with drying
salt, or molsieves, or by using toluene as the solvent, so the water and the toluene form an azeotrope) from the reaction before the imine is
reduced.”
The statement above from Rhodium by Laptop says that the reduction of the ketone to alcohol is possible (this could be a problem) but I assumed the
path imine to amine is thermodynamically preferred (just a guess). They also say that water has to be removed from the reaction prior reduction but I
think that reduction can be done simultaneously so the equilibrium ketone to imine is moved towards the imine as it is reduced to amine making the
reaction irreversible.
“More theory: the reductive amination of the ketone can lead to higher amines but this can be overcome with the use a five times molar excess of
methylamine.”
John Payne at Rhodium’s said that is necessary methylamine in excess… Ok.
Barium has an article where he says the “wet” amination is possible using NaBH4. It is a good work but I think the use of toluene makes necessary
a real good stirring and the imine formation may not be completed when the fases are separated lowering the yields.
So, let’s work: I will use cyclohexanone.
Cyclohexanone 20 g (0.21 mol) is mixed with 100 ml MeOH and stirred (magnetically) and put to chill using ice bath ( temp 0 to 5 Celsius all the time
), next, at time 0, 30 ml methylamine 40% (0.39 mol) is added. After 30 mim the first portion of NaBH4 (5.0 g (0.13 mol) divided in 7 portions) was
added and it was repeated each 15 min so it will end at 2 hours and the reaction was left to stir for more 30 min. Samples to HPLC were taken at 30
min, 1 h and 1 h30 min. The reaction was ended putting the flask directly to a rotovap and distilling off the methanol.
After that, 150 ml H2O was added and the pH was adjusted to pH 1 with HCL 50% with care. The liquid was then transferred to a separation funnel and
extracted with 3X50 ml DCM. The organic fases were put together and the DCM eliminated using a rotovap giving 5,2 g of a non basic residue.
The aqueous left was made basic with NaOH 30% (with care) to pH 13 and extracted with DCM 3x80 ml. The DCM extracts were put together and after
Rotovap left 15.4 g of an yellow oil with a characteristic smell, yield 64.9 % on cyclohexanone. After distillation under 20mmHg the pale yellow
liquid boiling at 66 - 68 Celsius was collected (13,3 g, yield 59,4 %). The distillate had n = 1.454 (lit. 1.455 to 1.454) and d=0.856 (lit. 0.86)
identified as n-methylcyclohexylamine.
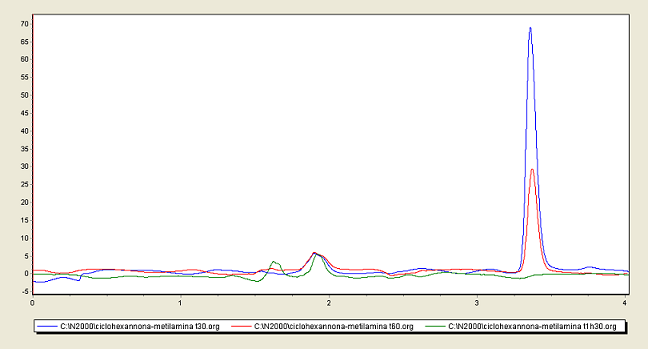
The reaction’s HPLC was running using ACN/H2O (70/30) 1.0 ml/min using a symmetry C18 at 30 celsius, readings was done at 282 nm. The samples were
50 micro liters diluted in 1.0 ml ACN injected 20 micro liters.
As we can see after 1 h 30 min there was virtually no more ketone.
The same process was repeated with 25 ml ethylamine 70% and the results were: non basic residue 5,0g, amine after distillation 14,1g , yield 52,9% of
pure n-ethylcyclohexylamine.
I am much sure that the process can be used with other more “nobles” ketones and the results will be very satisfactory.
I hope it can help someone or at least begin a good discussion about “wet” reductive amination. Greetings to you all.
[Edited on 4-4-2012 by DrNoiZeZ]
[Edited on 4-4-2012 by DrNoiZeZ]
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Wow, you have HPLC available and NaBH4 and are able to use it but cannot get dry methylamine from 40% aqueous and you regard 52% as a satisfying yield
in this reaction? And mostly ancient reverences are cited albeit a search here would produce much more up to date information on this really very
common topic?
Life is unfair 
And I hereby call for the foundation of:
THE SOCIETY FOR THE VINDICATION OF MERCURY
Using NaBH4 to dehydrate reaction mixtures is a policy which sadly is to expensive for me, not to to talk about the waste of a precious reducing
agent. To run a reaction which gives under anhydrous conditions 90%, under not anhydrous 62% more or less in water to get a lousy 52% and claim this
as success for being lazy is at least questionable and not good chemistry.
Maybe I overlook something here, then pls wise me up as I am pretty confused what this is about. Was it not yields we want?
/ORG
PS: The Al/Hg gives 85% and more and workup is not bad if done right and no unnecessary excess of Al was used (in cases the product steamdistills
workup is cake anyways).
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Organikum  |
Using NaBH4 to dehydrate reaction mixtures is a policy which sadly is to expensive for me, not to to talk about the waste of a precious reducing
agent. |
Actually he's not really dehydrating (as in drying) the reaction mixture. He would have to use much more NaBH4 for that.
The problem is not that the water destroys the reducing agent. It's rather that the equilibrium
ketone + amine <=> imine + water
is shifted to the left and in consequence the ketone is reduced to the alcohol, which can not form an amine. That's why in labs which can afford it
they use the cyanoborohydride. It doesn't reduce the alcohol, but the imine.
I think this was an interesting contribution, although in the same situation I would probably try to do the dry methylamine gas + silicagel thing.
Working with gases is not so bad if you know what you are doing.
PS: I'm a great fan of mercury as well. Though maybe not of the dissolving metal reductions. What a terrible mess, but for us amateurs often the
lesser evil. 
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
1- wow yes I have HPLC and NaBH4 and Hg and all you can think and I have the right to try anything the way I want
2- yield 50% is good to me since I can do the reaction in 3 hours and Hg:Al can be good to you but not to me because it is a mess and last 6 hours
(for me) and my yields are not 90% like you but some like 15%
3- I searched a lot and could not find good references about the wet method using tolueno, so I decided to CHANGE and I think it is the spirit of
science
4- Once I never had any information about that way of do it I shared it here with others to have the feedback like you did
5- concerning the Mercury I don't like to handle it, it is dangerous and you know that, so I decided to stop using it
6- to finish, yes I could handle anhydrous methylamine but why For me it is safe to do that way with 40% solution, sorry if I dont think the way you
do but I have my reasons.
Thanks for your information we will be always here for the best
[Edited on 4-4-2012 by DrNoiZeZ]
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
Now Turd is talking my language, thats the point equilibrium and I tried to demonstrate that, that's chemistry
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
The problem is not that the water destroys the reducing agent. It's rather that the equilibrium
ketone + amine <=> imine + water
is shifted to the left and in consequence the ketone is reduced to the alcohol, which can not form an amine. That's why in labs which can afford it
they use the cyanoborohydride. It doesn't reduce the alcohol, but the imine.
But I want to say that it is not the way it happens, I know that there is the possibility to reduce the ketone to alchool but it didn't happen too
much, from 20g of ketone 5g were not reduced to amine, it is a good point, the reaction works and it is easy. There are others ketones that I used and
the yields where above 70%. The NaBH4 reduces the imine too and well. At least that was my experience.
|
|
|
ripple
Harmless

Posts: 19
Registered: 19-1-2012
Member Is Offline
Mood: No Mood
|
|
everyone take a deep breath. sheesh, this place is like an anime convention with the heat cranked up. Picking a fight over yields, and responding with
an itemised list of defensiveness? Are we in the middle-east region of the science world?
1. Stop the nerd-on-nerd violence!
2. Your mom.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
i consistently get very dry alcoholic solutions of methylamine by dropping concentrated NaOH onto methylamine hydrochloride.
if i used another addition order i got heat output which drives off water.
so this order has served me well.
remeber to use a gas dispersion fitting and a suckback trap.
the main thing to pay attention to is the temperature of your alcohol (because methylamine dissolves exothermically) and the rate of gas absorption
has to match the rate you generate it.
it takes trial and error to get it right but once you get it down you can bang it out, batch after batch.
[Edited on 5-4-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
DrNoiZeZ, thanks for sharing your results. Now that you have the methodology in place, could you invest some time into one more experiment? Only if
you have some personal interest into doing so. Specifically:
Let a suspension of Na2CO3 (3 eq.), methylamine hydrochloride (1.86 eq.), and cyclohexanone (0.21 mol) in methanol (100 mL) stir for 24 h at RT. Then
at the usuall conditions add the usual amount of NaBH4 in the usual manner and proceed exactly as you did before.
This should put some light on the influence of water on the reaction yield and selectivity.
While the reductive aminations of aldehydes with NaBH4 are commonplace, there are only few reports of such a reaction on the ketones which form imines
in a less favorable equilibrium. Therefore, I think DrNoiZeZ's contribution is a precious one and we can forgive him if he did such a poor job in
reviewing the pertaining literature.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
THE SOCIETY FOR THE VINDICATION OF MERCURY
sign me up! - HgCl2 is cheaper than STAB and NaCNBH3 (please note stoich.)
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
"Let a suspension of Na2CO3 (3 eq.), methylamine hydrochloride (1.86 eq.), and cyclohexanone (0.21 mol) in methanol (100 mL) stir for 24 h at RT. Then
at the usuall conditions add the usual amount of NaBH4 in the usual manner and proceed exactly as you did before."
Sorry for the delay I was without internet
It seems interesting Nicodem, I will begin today, think in two days I'll have the results.
[Edited on 9-4-2012 by DrNoiZeZ]
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
After the 24 hours, the suspension was filtered with vacuum and the filtrate was washed with more 50 ml MeOH, all the liquids were put together and
them 5g NaBH4 was added in portions every 15 mim, rotovap to exclude the MeOH , 100 ml dH2O added, acidified with HCl, extracted with DCM giving a
residue non basic (2,5g), the acid solution was neutralized to pH 13 with NaOH and them extracted with DCM giving 16,4 g of an oil that was distiled
at 20 mmHg giving 14,6g (yield 65,2%) of a clear oil identified as n-methylcyclohexylamine.
I really expected more, but I could have some loss when I filtered the Na2CO3 even washing it with with MeOH.
Anyway it seeems to work well. Thanks.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thank you very much for your effort.
I don't think the filtering was necessary - those solids would unlikely interfere with the reduction. The difference in yields is indeed small (though
65% can be acceptable for preparative use), which means the water is not terribly detrimental for the imine formation in this particular case. This is
kind of comprehensible in the view of cyclohexanone being quite electrophilic for a ketone and so is methylamine for an amine. For sterically more
demanding acyclic aliphatic ketones and higher amines, water content might have a much more dramatic effect than it had for this specific pair of
reactants.
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
Thanks.
As I said: at least a good discussion.

|
|
|
Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
Another reducing reagent that has pretty much replaced NaCNBH3 is sodium triacetoxy borohydride. This avoids the HCN hazards and is amenable to scale
up. I don't know the review article in JOC that was published some years ago on its use, but you ought to be able to find it. I think the main author
was Marionoff from Johnson and Johnson.
|
|
|
nyll
Harmless

Posts: 7
Registered: 20-4-2012
Member Is Offline
Mood: No Mood
|
|
You can also use zinc dust instead aluminium in the Al/Hg method. It has the advantage that zinc does not need to be amalgamed with nasty mercury
salts like aluminium and can be used as it is, besides being easy and cheap to acquire.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Quote: Originally posted by nyll  | | You can also use zinc dust instead aluminium in the Al/Hg method. It has the advantage that zinc does not need to be amalgamed with nasty mercury
salts like aluminium and can be used as it is, besides being easy and cheap to acquire. |
I've read the paper, I'd like someone's experience
ʃ Ψ*Ψ
Keepin' it real.
Check out my new collaborated site: MNMLimpact.com
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by nyll  | | You can also use zinc dust instead aluminium in the Al/Hg method. It has the advantage that zinc does not need to be amalgamed with nasty mercury
salts like aluminium and can be used as it is, besides being easy and cheap to acquire. |
This is a science forum, so you are expected to give references.
|
|
|
DrNoiZeZ
Harmless

Posts: 45
Registered: 10-9-2011
Member Is Offline
Mood: No Mood
|
|
I remember to read some work from a Brazilian publication about that method using Zn and acidic conditions (as I can remember). I should have it at
home if I'll find it I'll give the reference.
As I said there are many ways to make that reduction.
Thanks.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DrNoiZeZ  | | I remember to read some work from a Brazilian publication about that method using Zn and acidic conditions (as I can remember). I should have it at
home if I'll find it I'll give the reference. |
You probably mean DOI: 10.1016/j.tetlet.2007.08.092 (Brazilian authors and zinc) where the N-methylation is described by the reductive amination of
formaldehyde using Zn in an acidic buffer. However, only formaldehyde is described and there are no reductive aminations of ketones described there.
I know of one such article (Synthesis 1991, 1043), but it describes a method limited to aromatic amines only or as the authors conclude:
| Quote: | | Aliphatic amines failed to give the desired product, although they formed the corresponding imines under the reaction conditions. Apparently, these
imines do not undergo reduction with zinc/acetic acid. |
That's why I would like to see articles of the reductive aminations of ketones using zinc, possibly working also on aliphatic amines (with in situ
imine formation). Currently, I'm only aware of a patent for the reductive aminations of ketones and aldehydes using Zn and catalysis by nickel salts.
Otherwise, preformed imines readily reduce with zinc in basic media (e.g., with Zn and aq. NaOH as in DOI: 10.1016/S0040-4039(98)01904-2), but this is
limited to stable and isolable imines.
Slightly on topic: The reductive amination of ketones using magnesium is also known (J. Chem. Soc. Perkin I, 1995, 265).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Palladium
Harmless

Posts: 30
Registered: 17-1-2011
Member Is Offline
Mood: No Mood
|
|
Reductive amination of ketones under aqueous conditions works with both primary and secondary amines. According to US Patents, the primary amine must
be made from its organic salt of ammonia if satisfactory results is expected. With straight ammonia, it should be anhydrous.
The secondary amine is often made with 40% aq-solution of methylamine with Pt.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Palladium  | | Reductive amination of ketones under aqueous conditions works with both primary and secondary amines. According to US Patents, the primary amine must
be made from its organic salt of ammonia if satisfactory results is expected. With straight ammonia, it should be anhydrous. |
Which US patents? You don't give any references. Are you talking about the original topic (reductive aminations of ketones with NaBH4) or some other
type of reductive aminations? Are we supposed to read your mind? 
|
|
|
Palladium
Harmless

Posts: 30
Registered: 17-1-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | Quote: Originally posted by Palladium  | | Reductive amination of ketones under aqueous conditions works with both primary and secondary amines. According to US Patents, the primary amine must
be made from its organic salt of ammonia if satisfactory results is expected. With straight ammonia, it should be anhydrous. |
Which US patents? You don't give any references. Are you talking about the original topic (reductive aminations of ketones with NaBH4) or some other
type of reductive aminations? Are we supposed to read your mind? 
|
I'm purposing on reductive aminations in aqueous conditions. Maybe I have missed that this "wet reductive amination" must be performed with NaBH4. In
the latter case I have nothing to say.
Some other writers noticed that this thread was waste of time, recently I did also.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Palladium  | | I'm purposing on reductive aminations in aqueous conditions. Maybe I have missed that this "wet reductive amination" must be performed with NaBH4. In
the latter case I have nothing to say. |
I was not limiting you in any way. You are welcome to make a review of any type of reductive aminations you want. But you gave nothing, not even a
single reference and no comprehensible information. It was thus expected that some annoyingly curious reader (like myself) would reply with questions
asking for clarification. Why don't you simply answer those questions?
| Quote: | | Some other writers noticed that this thread was waste of time, recently I did also. |
Just because you don't understand something, it does not mean it is a waste of time. DrNoiZeZ provided very interesting experimental results. What did
you provide?
|
|
|
Palladium
Harmless

Posts: 30
Registered: 17-1-2011
Member Is Offline
Mood: No Mood
|
|
I understood, and knew the comment with reference to reductive amination of a ketone in the prescence of water.
www.google.com/patents/US3187047.pdf
Please give us some comments about why the water is added intentionally here (GAA/-OH dissolves the ammonium acetate well enough), but in the case of
only ammonia in -OH solution water is more or less devastating.
|
|
|
| Pages:
1
2 |