LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
Beryllium Toxicity
I recently purchased 1 gram of beryllium from Gallium Source. The website states that no dust or powder is present. How safe is it to keep this in an
unsealed container? Is aqueous beryllium chemistry any more dangerous than, say, aqueous cadmium chemistry?
hibernating...
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i have a couple of samples of Be i keep it in a sealed bag but as long as it is not in powder form and you wash your hands after handling you should
be fine .
breathing the powder could lead to berylliosys (spelling?)
and the carbonate taste very sweet it used to be called glucinium(never tried to taste it though i trust my books..) but is very toxic as a metal and
as a salt (oxide, chloride....)
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
The stuff doesn't really oxidize readily in air, though I do have some grayish fingerprints on mine. There's probably a skin of oxide, but I don't see
it disintegrating into carcinogenic powder anytime in the near future. Mine sits out on a shelf.
I wouldn't bother doing any chemistry with it. Then you risk making finely divided carcinogenic stuff...and the chemistry looks rather boring. Nothing
obvious with colors. The oxide is amphoteric and the halides are rather covalent, reacting pretty readily with water. About the only neat thing is the
basic acetate, which has a neat cage structure
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
Ive heard a few stories from one of the organometallics professors on campus. One of them involved a colleague of his that worked with beryllium,
every time he was exposed he would start to bald. Then, after enough time for the beryllium to be excreted from his body his hair would grow back, so
good news is its not a cumilative toxin, bad news is you could go bald. It is an acute poison so high levels at once can be deadly. It is similar to
magnesium and can replace it in a lot of enzymes.
I found a google book exert here
http://books.google.com/books?id=nKulgztuzL8C&pg=PA423&a...
that talks about beryllium toxicity in more detail if that helps.
[Edited on 21-2-2012 by ThatchemistKid]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
You're ok. Beryllium doesn't react with air to produce volatile products. No sealing is neccessary unless it's a fine powder which can be dispersed.
Can you take a photo of it? 
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
I have a medium sample out on a shelf as well- Don't try and cut it, break it, or cause any particulates/salts to come from it, and you'll be fine!
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Endimion17  | You're ok. Beryllium doesn't react with air to produce volatile products. No sealing is neccessary unless it's a fine powder which can be dispersed.
Can you take a photo of it?  |
It's *exciting* stuff!
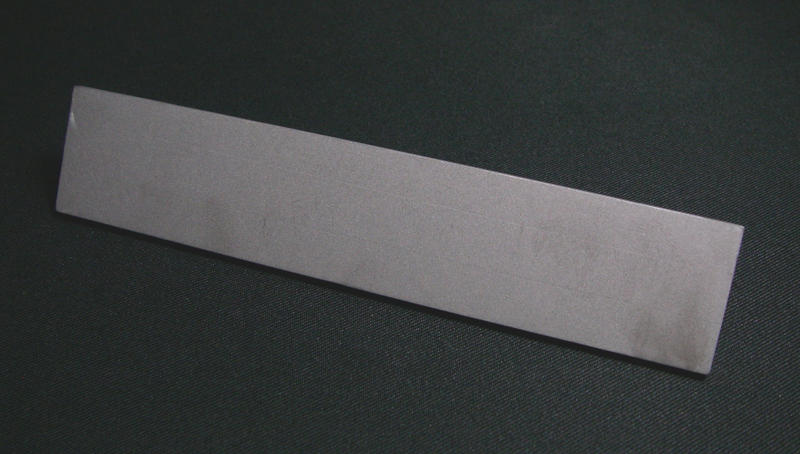

Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Quote: Originally posted by UnintentionalChaos  | The stuff doesn't really oxidize readily in air, though I do have some grayish fingerprints on mine. There's probably a skin of oxide, but I don't see
it disintegrating into carcinogenic powder anytime in the near future. Mine sits out on a shelf.
I wouldn't bother doing any chemistry with it. Then you risk making finely divided carcinogenic stuff...and the chemistry looks rather boring. Nothing
obvious with colors. The oxide is amphoteric and the halides are rather covalent, reacting pretty readily with water. About the only neat thing is the
basic acetate, which has a neat cage structure |
the only real exciting thing with Be is that it becomes a neutron source when expose to alpha particules of about 4 to 5 Mev.
but to get an efficient source of neutron (other than having a powerful source of alpha) it has to be mixed thoroughly with the alpha in a powder like
fashion and melted...hardly a home chemist/physicist weekend project...not impossible but very safety challenging
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
An interesting Chicago Tribune article on beryllosis in Manhattan Project workers:
http://www.ohiocitizen.org/campaigns/brush/chicago-bomb.htm
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
That's a cheeky stripe of metal you've got there. 
|
|
|
strontiumred
Harmless

Posts: 36
Registered: 6-7-2007
Location: Southern England
Member Is Offline
Mood: Delocalised
|
|
Just a word of caution - I bought some Be for my element collection and found I was allergic to it. Where I had touched it I had a red, itchy rash!
Cleared up after a day or so. I always wear gloves when handling it now.
|
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
This sphere is about 1 centimeter in diameter. It is quite dark gray and not very shiny.
   
hibernating...
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
looks just like mine! with the little crater and everything! guess we had the same suppier
|
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
Beryllium nodules often do have strange shapes.
hibernating...
|
|
|