| Pages:
1
2
3 |
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Lithium from Batteries
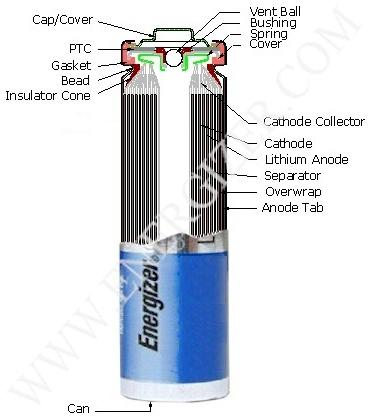
Today I managed to extract lithium from energizer batteries  Theres quite alot
of Li in there. It is not as scary as I thought it would be. I managed to cut open the battery AND remove enough of the case to pull out the coil
without shortcircuiting enough it to cause it to burst into flames. The net has no real documentation of dismembering Li batteries (Theodore Gray has one, but its kinda confusing), so I'll post one here Theres quite alot
of Li in there. It is not as scary as I thought it would be. I managed to cut open the battery AND remove enough of the case to pull out the coil
without shortcircuiting enough it to cause it to burst into flames. The net has no real documentation of dismembering Li batteries (Theodore Gray has one, but its kinda confusing), so I'll post one here 
Lemme get some lunch first though 
[Edit]: Procedure is here 
Anyone who's not foolish like me, will wear a pair of fireproof gloves. I did it barehanded (cause I didnt have fireproof gloves), but I dont
think thats something to be proud of 
Then get pair of diagonal cutters, and large pair of heavy duty pliers that can firmly grasp the battery, a small pair of long nose pliers, and the
optional stuff includes: a hack saw, oil, lotsa dish washing soap (a must if you're using the oil  ) )
DO THIS OUTSIDE IN A FIREPROOF PLACE!! 
I dont have any pics of the procedure cause I had to work kinda fast. Sorry guys 
First, peel off the wrapper of the battery. You'll notice theres a "neck" (that indented part) near the top of the battery and that
the negative terminal IS the ENTIRE CAN (see pic below).
If ANY part of the can touches the positive terminal, you'll see a tiny but bright spark indicating a short circuit. Because of this, we
can't open the battery like the conventional carbon batteries where you open and peel from the top. Instead, use the diagonal cutters to cut
the top off at the neck while holding the battery with the large pliers (like decapitating the thing  ). Do this as quickly as you can because you may be short circuiting the battery. If it continues to short,
it'll heat up really badly in a few seconds and may catch fire. Cutting off the top will remove all the stuff in the positive terminal and make
life a bit easier. If its too slippery to cut, use the hack saw make a nice rough spot to start cutting. ). Do this as quickly as you can because you may be short circuiting the battery. If it continues to short,
it'll heat up really badly in a few seconds and may catch fire. Cutting off the top will remove all the stuff in the positive terminal and make
life a bit easier. If its too slippery to cut, use the hack saw make a nice rough spot to start cutting.
Once the top's gone, you'll see a plastic ring on top of the inner coil (it's held in place by the remnants of the neck). Cut and then
peel away the can using the long nose pliers, starting at the severed neck. Peel off bits until you can easily pull out the coil. Do this carefully
and try not to dement the coil or touch the top of the coil, as that will shortcircuit the battery because the coil contains the anode and the
cathode.
Unwrap the outter most layer of plastic (its thin and has lines on it). If you want to keep the Li, grab onto a tad of the next piece of plastic (its
thicker than the previous one), then dunk the coil into oil and unroll the coil under there using chopsticks or something (its *not* an easy task  ). Most of the Li will be already dark brown, but some of it that was wound deep in
the coil will still be metallic. Some of the dark brown stuff in the coil is very thin and sticks to the plastic. It sinks in the oil and is not Li
(thats the iron sulfide and it turns dark green in water). The Li is the thick sheet that floats in the oil. Some other crap in the coil includes a
sheet of aluminum foil. ). Most of the Li will be already dark brown, but some of it that was wound deep in
the coil will still be metallic. Some of the dark brown stuff in the coil is very thin and sticks to the plastic. It sinks in the oil and is not Li
(thats the iron sulfide and it turns dark green in water). The Li is the thick sheet that floats in the oil. Some other crap in the coil includes a
sheet of aluminum foil.
[Edit 2]: Posted some pics 

Thats the top severed at the neck and the remains of the can after my peeling spree.

The unrolled coil under canola oil. Still some shiny Li floating there.

Lotsa shiny Li 

Heres the Li foil reacting with water
Cleaning up was NOT fun. I had oil everywhere and enough dishwashing foam in my yard to drown in it 
[Edit3]: I tried to make the procedure a bit more understandable.
[Edited on 7-7-2004 by Saerynide]
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
What type of solvent did you use for extracting the lithium?
N/A
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
No solvent is needed to extract Li, I just recantly aqquired 5 large Li batteries, the best way I have found of gutting them is to use make a
downwards slit for about a couple of cm from the top of the battery, and using long-nosed pliars to curl round the casing and peel it off like the
ring-pull on one of those old style corned beef cans.
The problem I have, is not getting the Li out of the battery, but finding something to store it in that it doesn't float to the top
of it 
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
I dont think there is any thing we can use that it wont float on. It has a density of only 0.54ish 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
I am thinking of glueing a long glass rod to the lid of a jar, with a section of a sieve or somesuch mesh spread out over it, i think that would do a
good job of keeping the lithium down without displacing too much motor oil.
Great pics Saerynide 
Now all we need to do, is come up with a good way to melt it into blocks
Have you by any chance heard of calcium batteries? apparently they have a calcium metal alloy as one of the electrodes, I think maybe a thread on
battery chemistry would be a good idea, as there are just so many goodies to be had from batteries
[Edited on 7-7-2004 by Reverend Necroticus Rex]
[Edited on 7-7-2004 by Reverend Necroticus Rex]
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Mwhehehee.. Battery abuse is always fun (not to mention scary) 
Great idea about the lithium holding lid :d I'll do that with my next left over battery 
About melting the Li, Im not sure if thats a good idea. Read this other page of Theodore Gray's. Li tends to explode when melted in ceramic crucibles... Maybe glass will be safe?
And where can we get these calcium batteries?
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
Don't try melting lithium in glass, I have never actually done it, but from what I have heard, molten lithium has a nasty tendency to melt glass,
and produce a big hot fireball in the process
As for the calcium batteries, I found one scavenging on my local waste dump for a power source for my Na experiments
I never opened it up, but maybe somehow the Ca could be extracted from its alloy, as to how, I have no idea though.
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
If it's spent, I doubt there'd be any Ca left in it 
I dont think any of us should try melting Li 
And is it just me, or does Li smell really good??  Maybe its reacting with the
oil... Maybe its reacting with the
oil...
Hmmm... Im in such a good mood today 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
The dump near where I live has huge piles of quite well charged car and truck batteries, that's what I rely on for electrolysis, since I
destroyed my father's car battery charger
I wouldnt be too surprised if there was still a fair amount of Ca alloy to be had in those batteries
Interesting....Aparently the plates are composed of a Pb/Ca alloy, and the positive grids are at least partially made of silver
DAMN!
It looks like there is about 1% or so tops Ca in those batteries, a couple of percent Ag, and about 6% antimony.
Looks like a non starter, but I wonder if a sort of fine spongy lead couldn't be produced by dissolving out the Ca with acid, leaving a sort of
fine honeycomb-sponge structure.
[Edited on 7-7-2004 by Reverend Necroticus Rex]
[Edited on 7-7-2004 by Reverend Necroticus Rex]
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
I was wondering when you were going to do this, I almost U2U'ed you.  I
also have done this but I opened it on the wrong side not wanting any chance of shorting, primarily because my needlenose pliers have one tip broken
and I don't have many tools to handle it. When I did this, I had a plastic car wax top mostly filled with clear mineral oil (intended as a
laxative, with a bit of vitamin e, too) and I immersed the battery in it while working. If I needed to see it up close, I would use a dropper to keep
it soaked in oil, and of course I was wearing goggles. I learned soon enough that I couldn't use leather gloves for protection against heat, and
besides I would have no idea how hot it was until a spark or flame formed. I
also have done this but I opened it on the wrong side not wanting any chance of shorting, primarily because my needlenose pliers have one tip broken
and I don't have many tools to handle it. When I did this, I had a plastic car wax top mostly filled with clear mineral oil (intended as a
laxative, with a bit of vitamin e, too) and I immersed the battery in it while working. If I needed to see it up close, I would use a dropper to keep
it soaked in oil, and of course I was wearing goggles. I learned soon enough that I couldn't use leather gloves for protection against heat, and
besides I would have no idea how hot it was until a spark or flame formed.
I chose to use my mom's latex gloves (she's a nurse so she has boxes upon boxes of them  ) but after a while they'd soak up some oil and their pores would become visible. I was SOOO freaked out while
doing this, not wanting to initiate any undersired reactions. Everytime I did, by shorting or pressing the Li against the black stuff (I thought it
was MnO<sub>2</sub> but not sure), I would usually see a red spark and take the battery outside in a glass container full of oil as far
from buildings as possible and quit for the night. ) but after a while they'd soak up some oil and their pores would become visible. I was SOOO freaked out while
doing this, not wanting to initiate any undersired reactions. Everytime I did, by shorting or pressing the Li against the black stuff (I thought it
was MnO<sub>2</sub> but not sure), I would usually see a red spark and take the battery outside in a glass container full of oil as far
from buildings as possible and quit for the night.
I began at the bottom cutting sort of diagonally with an okay pocket knife, dulling it by the time I finished. Next, I cut another slit so that I
could grip the point with pliers and pull it to expose some of the innards. I kept pulling away the casing until I had no good place to pull. Too
bad it was only partly opened. I got the small Li strip out, but the much larger one was impossible without a small set of scissors to cut the
plastic layers so I could get to some Li each time. To say the least it was quite time consuming and I only got a small fraction out in a test tube.
My mineral oil allows Li to sink if there are absolutely no bubbles attached, which takes a while. Currently there are crystals forming on the edges
as sometimes the top would pop off due to hydrogen (I tried to add a droplet of water to test once and it seems to have sort of dissolved) and when it
comes off the 60%+ humidity can get in.
The battery still remains in mineral oil and I'm considering making lithium isopropoxide with some hopefully anhydrous isopropanol just to get
rid of it, and to be able to have an alkali alkoxide. I still need to make sure that reaction won't be too violent and won't catch anything
on fire. I have 7 more batteries to take apart "the right way", and the entire pack was bought for $20. The reason I took the first
battery apart wrong was because my internet connection wasn't very stable and I proceeded to do it anyway after weeks of waiting to see <a
href="http://www.rhodium.ws/chemistry/lithium.batteries.html" target="_blank">this page</a> to check the methods.
I had my twin brother always watch me in case I needed something or something really bad happened. I need to get better tools, but the ones I can
find at local stores are much too expensive. Perhaps I'll ask my mom or sister to buy me a couple of items. One time my sister came in while I
was doing it, and she was mostly fine with it. My older brother however came in later and I felt that I had to show him the reactivity of it so he
wouldn't go poking around (like he tends to do) and really hurt himself. By the way, there is a <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=1974" target="_blank">related thread</a>.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Oooohhh. Thanks for showing me the Rhodium page. That one always escaped my google searches.
I understand how freaked out you mustve been. I was terrified last time I tried it. As soon as my cutters touched it and I saw the first orange
spark, I stuffed the battery into a glass jar and called it a day. That was two weeks ago I think. But yesterday, remembering a line from Theodore
Gray's page: "Don't be put off by small fires", I just made myself do it 
It was acutally safer for me to not use gloves. I didnt have any gloves that were fire proof or that I could rip off easily should they catch fire.
I didnt use goggles either. Im so bad 
And there's no wrong way to dismember it. Its interesting how 5 people all did it their own different way. Pick which ever way works for you
 But I dont recommend Theodore's way though, his battery kept on catching
fire But I dont recommend Theodore's way though, his battery kept on catching
fire 
And you're lucky you had someone with you. IMy brother freaked and stayed far away from me as poss... He almost ran away from me when I showed him the Li He almost ran away from me when I showed him the Li 
About those crystals.... what *are* they? They remind me of how people describe AP. Are you sure they're not peroxides or anything dangerous
like that? 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
I always refer to rhodium's site if I'm wondering about some reaction. Particularly if you search for "lithium" the first hit
will be the battery deconstruction page. It's useful for more than drug synths. I remember the time I read and reread the birch reduction
document by mr clean there.
You seemed eerily laid back about the whole thing in your initial post than you are about anything, but at least I know the reason and how careful you
were being. Partly why I used gloves was that I kept the battery saturated with oil and bare-handed it would slip right through my fingers. Also,
sharp edges in the cut metal would mangle my glove and not my hand. I actually had a glove tear up pretty well when I tried to take it off one night.
Having a propylene carbonate and 1,2-dimethoxyethane solution of lithium perchlorate get in a cut is NOT my idea of having a good day. If my gloves
did need to be pulled off quickly, I could grip them at the wrist and yank them inside out on a moment's notice. It's not so easily done
with those kitchen cleaning gloves that are thicker.
I really doubt that those crystals are peroxides, because really only the other alkali metals form them upon combustion. It's probably just a
mixture of oxides and hydroxides, and nitrides if they're brownish black. This would be yet another reason why I would rather deal with lithium
than something else. I think it would take rather high pressures of pure oxygen to coax lithium to form peroxides, if it can even be done at all.
<a href="http://www.chemguide.co.uk/inorganic/group2/reacto2.html" target="_blank">This page</a> down where it says
"Why do some metals form peroxides on heating in oxygen?" explains why some metals form peroxides and others don't (yes they talk about
alkaline earth metals but the point is also valid for alkalis, too).
Next time you do this I would recommend having a friend watch you in case something like a burning molten bead of lithium decides to embed itself in
your arm when the thing catches fire. I had my twin wearing goggles and gloves and being just as scared as I was. I was doing it inside a store room
that gets a bit of air conditioning sometimes just so I would be working in a less humid environment. I wasted a good deal of the metal testing
whether it really was it or not, and even burned a small strip of it. I wore thick thick cloth gloves and it took a while to ignite due to the oil
coating but burned very quickly and with a spectacular red flame with much oxide being thrown into the air.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
In your last picture, the lithium doesn't seem to be reacting with the water very violently. No where near as violent as sodium. Do you think
there would be a way to synthesize lithium carbonate from the elemental lithium in those batteries?
[Edited on 9-7-2004 by tom haggen]
N/A
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
As the lithium is in a thin foil, you could probably get away with burning strips of it in a chlorine atmosphere, what are you using the LiCL for?
Bugger, sorry, I misread your post as wanting to make LiCl
[Edited on 9-7-2004 by Reverend Necroticus Rex]
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Lithium carbonate would be an easy one:
2Li(s) + 2H2O(l) ----> 2LiOH(aq) + H2(g)
2LiOH(aq) + CO2(g) ---> Li2CO3(s) + H2O(l)
Filter off your slightly soluble Li2CO3
Or evaporate your LiOH solution and leave the solid out exposed to the atmosphere, it will pick up CO2.
BTW: Good job Saerynide, any future plans for you Li? Oh, and melting in glass, not a good idea, I mentioned it somewhere that molten lithium redily
attacks glass emitting green light and melting holes quickly in the glass (possibly with explosion) as it does so.
[Edited on 7/9/2004 by BromicAcid]
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
So reacting lithium with water gives you lithium hydroxide?
N/A
|
|
|
sanity gone
Harmless

Posts: 38
Registered: 25-4-2004
Member Is Offline
Mood: No Mood
|
|
reacting metal or metal oxides that can displace hydrogen from water will give you the hydroxide.
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
Reacting any alkali metal with water gives the hydroxide, they skid about on water and from Na up they usually burn, Li skids about and fizzles on
water, Na does the same, but is a lot more likely to catch fire.
Na will explode if sufficient amount be present, K is VERY likely to explode,
I havent personally seen Rb and Cs hit water, but from what I have heard, explosion is a certainty and said explosion is extremely violent
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
sanity gone
Harmless

Posts: 38
Registered: 25-4-2004
Member Is Offline
Mood: No Mood
|
|
Try some Fr on water, that would be violent. Too bad that there is like no Francium in the world 
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
A chunk of francium reacting with water has been one on THE experiments I would most like to see, second only to maybe nitrogen tri-astatide
And I don't think "violent" would be sufficient to describe it......
[Edited on 9-7-2004 by Reverend Necroticus Rex]
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
| Quote: | Originally posted by tom haggen
In your last picture, the lithium doesn't seem to be reacting with the water very violently. No where near as violent as sodium.
|
Thats cause I only put about 5 drops of water on it. I didnt want to waste it all 
| Quote: | Originally posted by blip
You seemed eerily laid back about the whole thing in your initial post than you are about anything, but at least I know the reason and how careful you
were being. |
Sorry if it sounded like I didnt care about anything. Maybe it was the effect of the Li (the Li+ ion messes with your mind). I found myself in a
really-happy-for-no-reason-while-not-thinking-about-anything kind of mood for a few hours. You dont know how hard it was to type that first post
sensibly 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Is the lithium obtained from those batteries is contaiminated with lead, or any other toxic chemicals? Besides lithium itself being toxic in high
concentrations in the blood stream.
[Edited on 9-7-2004 by tom haggen]
[Edited on 9-7-2004 by tom haggen]
N/A
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I believe electrolytic lithium used in making lithium batteries is of an unusually high purity. I remember reading something on the incredibly
stringent measures put into purifying the lithium and keeping the rooms of production clean. So I don't think there is any note worthy lead in
the lithium obtained from batteries.
Any other contaimination would be surface contamination and removed by washing the freashly removed lithium strips with oil then acetone.
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Saerynide, do you think the happy mood you got was because of the Lithium? How much would a person need to ingest to cause that? You were not
touching the Li with your hand, were you? 
This sounds like a lot of fun, got to try it, and I will find my OWN way to open the battery.
Good, other people have found Gray's site. It's great.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
500mg would be a good healthy dose of lithium. You would be better of synthesizing lithium carbonate seeing as how that’s the actual medication
doctors prescribe. I would be careful when ingesting internal components of a battery. It just seems like a bad idea.
I find it highly unlikely that someone absorbed a large dose of lithium through skin absorption.
[Edited on 9-7-2004 by tom haggen]
N/A
|
|
|
| Pages:
1
2
3 |