gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
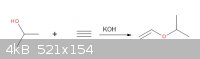
Acetylene + 2-propanol
Good Day, I have 3 questions
1)Is this scheme correct
2)If yes, what are the conditions of this reaction.
3)And what are the presumable properties of the product.
Thank you
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
I Cannot figure out a mechanism or conditions to make that work, I searched through some radical reactions with AIBN and Bu3SnH etc, nothing that
gives the radical on the oxygen causing it to then reaction with an acetylene or a double bond, the O-H bond is just too stable that its formation
competes with any other reaction and its rate is seemingly much faster.
Usually though such molecules can be accessed through enolates, captured enolates such as silyl enol ethers or even possibly an enolate reacting with
with an electrophilic carbon with HMPA as an additive in the reaction mixture.
Silyl enol ethers are generally made by reaction of the ketone with a base such as LDA or KH, other more or less hindered lithium amide bases,
depending on whether you want the thermodynamic or kinetic enolate and whether you want the E or Z isomer of your enolate. after the enolate is formed
it is reacted with a trialkyl silyl chloride usually such as TMS-Cl,
if you want to read more about silyl enol ethers a quick stop to the wiki page and http://www.inchem.org/documents/jmpr/jmpmono/2001pr07.htm#2....
might be helpful.
in your case, you would have to react the enolate of acetyl aldehyde ( so there is distinguishing E/Z isomers and thermo/kinetic products, which is
always a plus) with a carbon electrophile such as 2-propyl bromide and Hexamethylphosphoramide, in DMSO or DMF (HMPA as well as DMSO and DMF are used
to relieve aggregation of the negatively charged oxygen formed and the lewis acidic cation counterion of the base used via chelation to the cation,
thereby making the oxygen more open to nucelophilic attack, essentially affecting O-alkylation vs C alkylation). This presents many problems,
enolization of acetyl aldehyde tends to give lots of crap polymerization (though you might be able to use acetyl aldehyde generated from paraldehyde).
Reaction with LDA may afford reduction products because of a tendency of aldehydes to undergo a reaction akin to the Meerwein-Ponndorf-Verley
reduction with LDA ( http://en.wikipedia.org/wiki/Meerwein-Ponndorf-Verley_reduct...). Also your carbon electrophile is a secondary carbon and you will have
elimination by-products.
there are other methods to get to what you are looking for. Do you have a proposed mechanism for your reaction,
(maybe one that is a large oversight on my part)?
Where did you get the idea to do this and what for?
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
Foot in mouth! actually you can form that product from those reactants using chemistry known as Reppe chemistry.
http://en.wikipedia.org/wiki/Walter_Reppe#Reppe_chemistry
I know nothing about this, though it seems you need a metal catalyst and high pressure to afford the transformation, not commonly had at home.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Reppe chemilstry is still used industrially though less than in previous times as acetylene has fallen out of favour as a starting material.
Organometallic courses tend to focus on newer and sexier catalysts and processes.
But it is still a good way to make some things eg cyclooctatetraene aka COT which is a popular ligand and starting material for other research.
http://en.wikipedia.org/wiki/Cyclooctatetraene
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
both my graduate organometallic courses ( one taught by an inorganic chemist and one taught be an organic chemist so I feel ill get a full picture of
the topic  ) are next quarter would you mind giving me some examples of the
catalysts used in the process? or some links or refs to stuff I can read. ) are next quarter would you mind giving me some examples of the
catalysts used in the process? or some links or refs to stuff I can read.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Organometallic chemistry is a vast field with lots of industrial examples.
Good industrial examples are;
SHOP http://en.wikipedia.org/wiki/Shell_higher_olefin_process
Ziegler- Natta http://en.wikipedia.org/wiki/Ziegler-Natta_process
Monsanto and Cativa http://en.wikipedia.org/wiki/Monsanto_acetic_acid_process
Thats enough processes - Ed 
[Edited on 16-1-2012 by ScienceSquirrel]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
There are conditions to give ethyl vinyl ether listed in organicum (sp? the old german practical organic text). IIRC the conditions are KOH in
alcohol in a bomb, with acetylene under moderate (<100 psi) pressure. I suspect the same conditions would work for isopropanol.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|