| Pages:
1
2 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Phenolic Sugar Alcohol nitration candidate
Yeah it is very interesting. The polydextrose is also interesting. Fructosan Trinitrate gotten from nitration of fructose is also reportedly stable.
Several of these sugar derived energetics would seem to be of interest but there is very little information on them to be found.
Here's the latest thing I have been looking at along the same general lines Phenolic Sugar Alcohols as candidates for nitration, especially
interesting is the reported condensation product of glucose and aspirin, and not specificially but generally mentioned other possible condensations
of glucose with salicylic acid, and methyl salicylate. What might be gotten from nitrations of these condensation products is unknown and could be
useful. What the disclaimer attached to the patent is about I am not sure,
whether the disclaimer means the claimed compound is misidentified or if it was discovered a prior art already claimed these compounds.
Attached also is the British patent by the same inventor which has some added information GB518586
It would be interesting to learn what is the reaction product of glucose and resorcinol and what may result upon nitration.
Attachment: US2252725_PHENOLIC_SUGAR_ALCOHOLS.pdf (334kB)
This file has been downloaded 953 times
Attachment: Fructose Trinitrate from Nitrocellulose_industry.pdf (183kB)
This file has been downloaded 750 times
Attachment: GB518586_Phenolic Sugar Alcohols.pdf (532kB)
This file has been downloaded 546 times
[Edited on 9-9-2011 by Rosco Bodine]
|
|
|
vaslop
Harmless

Posts: 12
Registered: 13-4-2011
Member Is Offline
Mood: No Mood
|
|
Im surprised that this compound hasn't taken off, has anyone tried making any glucose ureide?
I tried the synthesis as follows
10.2g of glucose hydrate dissolved in 3g of water at 60c.
34g of urea is added and stirred in.
When the temperature is 60c again, 91.8g of glucose hydrate is added and also stirred in.
Afterwards, once the mass is mostly melted, a mixture of 3g of water and 3g (~1.6ml) of sulphuric acid are added slowly.
The mixture reacts at 60c for 8 hours.
Here, no solid glucose ureide was noticed, however the black viscous mass was obtained, but I continued with the synthesis as is written in the
patent.
The mix was dissolved in very little water and neutralised with barium carbonate.
This was then filtered and evaporated, however no precipitate was obtained.
I have repeated this procedure but allowing a reaction time of 16hours, but with the same result.
Has anyone managed to make any glucose ureide yet?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There have been no experiments done by me on this yet but here is some food for thought in added references.
The monoglucosyl ureides are vulnerable to hydrolysis so precipitation using alcohol may be helpful. Isolation of the hydrolysis sensitive
monoglucosyl ureide in pure form may be most practical by first forming the hydrolytically stable glucosyl ureide - urea adduct which may be isolated
by crystallization from water, and then thermally decompsed by long refluxing in alcohol to yield the desired monoglucosyl ureide. This method for
isolation was described in an article attached earlier in the thread which reviewed the original work of Schoorl. The formation of the ureides is
favored by the presence of a dehydrating material or vacuum conditions and only moderately warm temperature. See GB653775 attached. Recent work has
shown the monglucosyl ureide forms in high yield from a melt using Amberlyst 15 as a catalyst. Possibly other acidic and dehydrating systems may also
be useful. A pyrophosphate and silica gel or the thermal dehydration product of ammonium bisulfate would seem to be candidate catalyst / dehydration
materials for a glucose and urea melt.
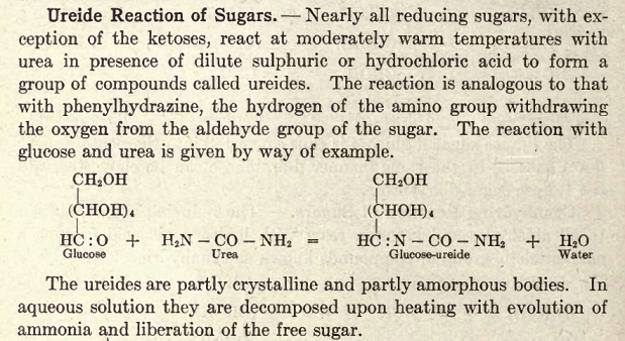
Attachment: GB653775 Glucose Ureide and Sugar Ureides.pdf (319kB)
This file has been downloaded 603 times
Attachment: Glucose Ureide Urea adduct biochemj01152-0221.pdf (490kB)
This file has been downloaded 627 times
Attachment: Glucose Ureide using Amberlyst 15 acidic catalyst.pdf (191kB)
This file has been downloaded 895 times
Attachment: Pages from handbookofsugara00browrich.pdf (139kB)
This file has been downloaded 951 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Something which may be a worthwhile experiment is to see if the glucose ureide would form via reaction of anhydrous glucose with the anhydrous Calcium
Nitrate and Urea adduct Ca(NO3)2 - 4(NH2CONH2) perhaps with some catalytic acidity provided by glacial acetic acid. The formation of the glucose
ureide is a dehydration type of condensation reaction favored by the presence of something which can remove the byproduct H2O and anhydrous Calcium
Nitrate is capable of absorbing H2O and can form a tetrahydrate which would precisely absorb the byproduct H2O from formation of the ureide. Perhaps
some eutectic melt system having added nitrates or other materials would be advantageous for the reaction scheme. Using alcohol or perhaps DMSO as a
solvent may also help. Urea nitrate is acidic and might also serve as an acidic catalyst.
US1911580 Example #2 shows a Calcium Nitrate / Calcium Nitrate - Urea adduct low melting point mixture which is of particular interest as it can be
vacuum dehydrated at 112C
to 0.7% H2O without decomposition of the urea to biuret or cyanuric acid. It seems possible that addition of anhydrous glucose or perhaps even
glucose monohydrate to such a melt, possibly with or without an acidic catalyst could form the glucose ureide adduct.
Interesting also is the Magnesium Nitrate - Urea addition compound which is commonly a dihydrate but may occur also as an anhydrous form. I am
looking for more references on this. Attached is one reference and a link for a synthesis
of the dihydrate in alcohol as well as from water.
http://pubs.acs.org/doi/abs/10.1021/ja01282a040
It may be feasible to form the glucose ureide in a nitrate melt and then to nitrate in situ by addition of sulfuric acid.
If such a scheme is workable the process would be greatly simplified. In such regard, the magnesium nitrate adduct
would be interesting due to the soluble sulfate byproduct
which would not complicate isolation of the nitrated organic material.
Attachment: US1911580 Calcium Nitrate Urea Adduct.pdf (118kB)
This file has been downloaded 861 times
Attachment: Thermal Stability of Urea Magnesium Nitrate Complexes.pdf (210kB)
This file has been downloaded 686 times
[Edited on 6-12-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by vaslop  | Im surprised that this compound hasn't taken off, has anyone tried making any glucose ureide?
I tried the synthesis as follows
10.2g of glucose hydrate dissolved in 3g of water at 60c.
34g of urea is added and stirred in.
When the temperature is 60c again, 91.8g of glucose hydrate is added and also stirred in.
Afterwards, once the mass is mostly melted, a mixture of 3g of water and 3g (~1.6ml) of sulphuric acid are added slowly.
The mixture reacts at 60c for 8 hours.
Here, no solid glucose ureide was noticed, however the black viscous mass was obtained, but I continued with the synthesis as is written in the
patent.
The mix was dissolved in very little water and neutralised with barium carbonate.
This was then filtered and evaporated, however no precipitate was obtained.
I have repeated this procedure but allowing a reaction time of 16hours, but with the same result.
Has anyone managed to make any glucose ureide yet? |
I have been further studying the literature and doing some checking of the stoichiometry have discovered what I am reasonably certain is an error in
the patent which could account for the crystallization difficulty you have reported.
The quantities of glucose which you used are a scaled down proportion of the quantity reported in the Meijer patents US2612497 and GB653775, but the
patent has evidently a typographical error in the text portion which misidentifies the glucose as the hydrate or monohydrate when the weight quantity
indicates an equimolar stoichiometry for the stated weight corresponding with the anhydrous glucose. The same error appears in the US and the GB
patents where the glucose is misidentified as being the hydrate but the weight stated would match use of anhydrous glucose. This error could have
also been a transcription or translation error from the Dutch inventor, because the correction amount of additional glucose monohydrate which would
balance the equation stoichiometry is coincidentally a second portion of 1020 grams, which would have likely been "lost in translation" as a second
added individual portion ...if all of the glucose is indeed taken to be the monohydrate which is the commonly available form. To be clear, there would
be required three additions total of Glucose Monohydrate, portion 1=1020 grams, portion 2=1020 grams, and portion 3=9180 grams which would be
equimolar to 3400 grams of Urea. This would make the total glucose monohydrate needed 11.220 Kg. which is 56.66 moles.
For example 2 of the Meijer patents where sucrose is inverted in situ to provide glucose, considering an inversion effciency of 100%, 9.7 Kg of
sucrose is the correct amount
which would upon hydrolysis to glucose give 56.66 moles glucose.
Doing the stoichiometry it is discovered that the weight figures in the Meijer patents do not reconcile with the text.
These adjusted figures would seem logical as a place to
begin troubleshooting the process. What should be a
good indicator of success is a crystallizing out of the desired product and at some combination of proportions and conditions that should occur since
this is reported by many different sources for variations on generally similar conditions.
See example 1 of GB467749 attached as a comparison patent for earlier work / prior art, where the inventor is a German chemist with a phd, Dr. Kurt
Quehl, who identifies the same glucose ureide gotten from a simple fusion at water bath temperature of an equimolar mixture of glucose and urea. There
is some discrepancy however about the melting point determination of 117C for glucose ureide. Hynd reported a decade earlier the mp of 207C for
glucose ureide. What accounts for the vast difference in melting points between the two studies is unknown and mysterious.
Dr. Quehl describes the difficult crystallization of the glucose ureide so it is probably safe to presume an impure glucose ureide of depressed
melting point is obtained as the initial product. The 117C mp value reported by Dr. Quehl differs considerably from the earlier researcher Hynd's
reported melting point of 207C, (See Alexander Hynd article January 1926). The US2116640 patent by the same Dr. Quehl and concerning the same subject
matter is also attached. Also attached is US1979121 which is referenced within these patents and regards the formation of adducts of urea with metal
salts. The formation of adducts of urea with metal salts is also a dehydration type reaction, and the analogy being recognized is precisely what has
caused me to speculate that these reactions may be possible to be combined as a precursor to nitration.
Earlier in this thread a link was posted by franklyn to an article by Irving Goodman which tends to bear out the results of Hynd as being accurate
with a mp of 207C or 208C
for the pure compound glucose ureide.
Here again is the link
http://books.google.com/books?id=NDnNCOG6RWcC&lpg=PA215&...
See footnote #13 on page 216 of the Irving Goodman article.
Better sense is now possible to be made concerning the discrepancy in melting points as the compound of Dr. Quehl is identified as being a simple
molecular addition product of glucose and urea, absent the dehydration which leads to
glucose ureide.
Attached is a more recent article describing a variation on the Hynd synthesis which first forms the adduct of urea with the glucose ureide in order
to facilitate crystallization, followed by boiling with alcohol to decompose the adduct and yield the desired glucose ureide in pure form mp 207C.
An excerpt from CA670146 which describes a variation on the Meijer synthesis using precipitation by methanol of the glucose ureide is also attached.
The product identified by its optical and melting point properties appears to be the desired monoglucose ureide. Perhaps adjusting the proportions to
equimolar would be an improvement as well as incorporating the precipitation using methanol. This synthesis can probably be further optimized with
some fine tuning of the process, which is better understood after a study of these references.
Attachment: GB467749 Glucose Ureide by Fusion of Glucose and Urea.pdf (266kB)
This file has been downloaded 575 times
Attachment: US2116640 Glucose Ureide by fusion of Glucose and Urea.pdf (159kB)
This file has been downloaded 574 times
Attachment: US1979121 Metal Salt Urea Adducts.pdf (94kB)
This file has been downloaded 521 times
Attachment: Glucose Ureide Synthesis.pdf (751kB)
This file has been downloaded 708 times
Attachment: Pages 10-11 from CA670146 Example 1 Glucose Ureide Synthesis.pdf (95kB)
This file has been downloaded 518 times
[Edited on 11-12-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
After considering the Example 1 of CA670146 attached above, which describes a synthesis of Glucose Ureide derived from the Meijer patent, further
thought has been given to the discrepancy of weights which I first believed may be a translation issue with the Meijer patents. I no longer believe
that such issue necessarily exists and the weights and description by Meijer may be valid as given. What appears to be an equimolar excess of urea
with regards to the glucose is offset by the sulfuric acid which ties up the excess of urea as urea sulfate. When that neutralization of the sulfuric
acid by the urea is taken into account, there is actually a 1 mole excess of glucose with respect to the available urea, so the weights given by
Meijer in the patents are reasonable and in fact do reconcile. The CA670146 Canadian patent excerpt which was attached in the preceding post seems to
be the most straightforward method found thus far for producing the Glucose Ureide.
In an earlier post I had mentioned the possibility that Urea Nitrate being acidic may suffice as an acidic catalyst, so I should have been more aware
of this function being produced in the reaction conditions described by Meijer where
the urea sulfate and possibly some urea acid sulfate is formed in situ. Earlier
I speculated it may also be possible other acidic salts such as bisulfates or pyrosulfates or pyrophosphates may also work as the acidic catalyst.
The additional process details supplied by the York patent CA670146 along with identifying the urea proportions must account for a neutralization
equivalent
of urea used to form in situ the urea salt of the acid catalyst provides confidence
that the process issue reported by vaslop who followed the incomplete description
of the Meijer patents can be resolved, and a crystalline product of high purity
should be obtainable.
The inventor who describes further details for the Meijer patents is the same William C. York as is inventor in the Diglucose Ureide patent which was
posted on the first page of this thread, and is inventor with several related patents assigned to W.R. Grace Company concerning derivatives which are
useful as detergents. York references the Meijer patents in his own patents which seem to be reliable, so it is reasonable to place confidence in the
process described
by York in example 1 of CA670146.
Worth noting is that Yorks observation of experiments more involved with Diglucose Ureide rather than Monoglucose Ureide as is our interest, is that a
minimum presence of H2O was required for the reaction to proceed .....and this may also hold true for the Monoglucose Ureide as well, which would seem
to contradict the expected reaction dynamic for a reaction that is a dehydration type of condensation.
As counterintuitive as this may seem it could be true that
an optimal amount of water is best also for the Monoglucose Ureide synthesis. In whatever case, the process as described by York in CA670146 should
resolve the issue
reported by vaslop.
[Edited on 12-12-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Some further indication has been found in a reference which tends to point to the circa 1963 York patent CA670146 as likely being a valid method.
Attached is a circa 1960 article which describes the circa 1960 already published methods being tested, including the Meijer patent method upon which
the later York patent method is modeled very closely. The Meijer and York related work is more conforming with the economics of process chemistry for
bulk industrial needs of textile and detergent product applications, where the absolute purity and precison is lower than for biochemical or
pharmaceutical preparations. However, the absence of any significant depression of melting point below 207C tends to
indicate acceptable purity for the more direct methods such as the York - Meijer processes which avoid the long reaction times and the isolation of a
urea adduct as a precursor which
must be further processed to obtain the desired glucose ureide. It would seem then that the York patent CA670146 example 1 is likely the best
published method among the methods which I have reviewed, based upon the literature which I have found.
Attachment: Glycosylureas. Part I. Preparation and some reactions of D-glucosylureas and D-ribosylureas.pdf (496kB)
This file has been downloaded 677 times
|
|
|
| Pages:
1
2 |