| Pages:
1
2
3 |
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
The most dangerous, expensive and inneficient way of making HCl!
Bottomline:
Electrolysis of a NaCl solution gives, initially, H2 and Cl2. The mixture of these two gasses is ignited by a spark, giving HCl gas that is absorbed
by water giving a HCl solution.
You can get hurt or killed by chlorine gas (used in war as poison), by HCl gas (worse poison), by explosion of H2 or H2 Cl2 mix, or you can
get electrocuted by the high voltage. At least, is not very efficient: I could only make 20ml of weak acid after 20 minutes fiddling with the setup to
keep it working.

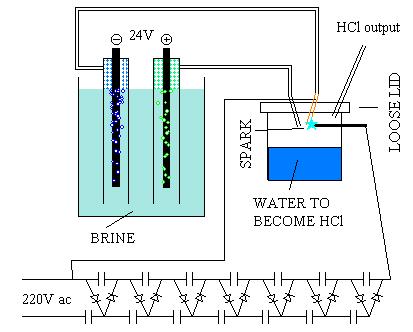

Making a short story long:
The electrode connected to the positive pole (anode?) has to be carbon (graphite). I used a solder’s carbon electrode. I had to strip away the
copper layer by electrolysis first. For the negative electrode I used copper wire. The electrodes are kept in PVC pipes with one end closed. If you
can’t understand why, you really are one little... aww, never mind.
Even with a strong brine and 24V (lots of current), not much gas is produced.
To produce the spark I used a Cockroft Walton voltage multiplier with 14 capacitors. I estimate in 3.000V the final output. Anyway, it was enough to
give a spark every second or so with a half millimeter gap. The tubes that inserted the gases in the spark chamber are made of brass and one of them
is connected to HV. A pencil lead graphite made the other electrode. THIS VOLTAGE MULTIPLIER MAY HAVE ENOUGH CURRENT CAPACITY TO KILL YOU! It
certainly has voltage enough! Edit2: By the way, the capacitors hold the voltage even after you turn off the power.
Don’t mix the gases before the spark chamber. The biggest explosion I ever had in my attic was last week testing another configuration. H2 and Cl2
make one hell of an explosive! edit1: This mix can be triggered by light! Use only soft plastic pots for the spark chamber, just in case...
I put some water in the spark chamber to absorb the HCl. I figure that a drying agent (instead of water) would allow the production of reasonably dry
HCl. Remember that, as the reaction goes on, NaOH is formed in the brine, producing oxygen, instead of chlorine and, therefore, water vapour instead
of HCl gas.
The plastic tubes that conduct the Cl2 and H2 are silicone rubber tubes. I bought those along with the thin brass tube in a hobby shop. You know, the
kind that sells models of aircraft and so. I guess these are used to conduct fuel for model engines. I presume silicone withstands chlorine better.
[Edited on 13-6-2004 by Tacho]
|
|
|
BromicAcid
International Hazard
    
Posts: 3254
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Rather then spark gap you could just pass the resulting gasses though a tube packed with activated carbon or pumice at 300C then pass the resulting
gasses though H2O. As it stands you say you made 20 ml of weak acid. Well, passing just Cl2 though water makes it weakly acidic, any quantitative
tests for HCl (however they would not be any accurate seeing the equilibrium formed between Cl2 and H2O)? If you made your solution significantly
concentrated then it would have to be HCl by default as only it gets to a significantly high concentration, especially in relation to Cl2.
One time my chemistry teacher was doing a demonstration and had a balloon full of H2/Cl2 to show the class the photosensitivity of the mix. But she
had to run from the building where the chemicals were stored to where the lectures were given, halfway there the balloon achieved the activation
energy it needed and detonated. Coincidentally it just so happned to be one of the days that we had a political figure on campus, a bad day for such
an occurrence to happen.
BTW, I expecially like the madness to your idea, high voltages, explosive gas mixes, it doesn't get much better then that.

|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
You must have a death wish  
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Saerynide
You must have a death wish   |
Not really. I am very, very careful.
I make all those comments about the dangers involved to make sure anyone repeating the experiment knows what he/she should be careful about.
The "biggest explosion" was just an extra- loud "pop" that caused no damage but brine on my benchtop and some plastic bits in my
lap.
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Do you need to use sparks to detonate the mixture? Why don't you just bring the mixture with water out to get some sunlight? sunlight?
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Sunlight may or may not detonate the mix. It’s not a 100% sure catalyst. Besides, it may detonate only when too much mix is in the pot and that
means an explosion.
The sparks are not a problem. The main problem with this procedure seems to be the chlorine. I think it may be dissolving in the brine instead of
bubbling out. If I put the output tubes under water, hydrogen’s bubbles a lot, but chlorine’s doesn’t. Just a little bubble now and then.
Edit: I know the volume of hydrogen should be quite bigger but, even then, something doesn't seem right.
[Edited on 14-6-2004 by Tacho]
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
The amounts of chlorine and hydrogen produced are to small for to make a noticeable reaction with the spark.
The glasstubes around the electrodes limit the amount of brine VERY badly. You will produce more oxygen than chlorine also I guess.....
this is some quick and dirty guessing of mine, no scientific anylysis.
ORG
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Those are not glass tubes, they are PVC pipes. They are 20 mm diameter and open in the bottom.
Under 24V there is a fair amount of hydrogen being produced, (edit) some chlorine that I can smell and there are small flames now and then in
the spark chamber. Fumes are produced. The water becomes acidic.
It works. It’s just not efficient.
Edit: I'll try larger tubes.
[Edited on 14-6-2004 by Tacho]
[Edited on 14-6-2004 by Tacho]
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
when I used 9v to electrolyse a saturated salt solution, I got a ton of Cl2. It took only about 5 min to fill a large tube and show a noticable light
green colour 
Try not inserting the tubes to deeply. I inserted mine deep enough only to cover a bit more than the electrode's length.
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
actually, HCl has/is made industrial in this way.
Cl2 is burned in H2atmosphere (excess) in special constructed quarts nozzles and cooled , absorbed into H2O.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
WAIT!, why am I saying that I should get less chlorine? No! I should be getting exactly the same volume of chlorine! One electron to every H+, one
from every Cl-! 22,4liters of gas for every mol of it!
Something wrong here! Maybe some copper was left in the carbon electrode and is depositing instead of chlorine!
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Some water is oxidized, instead of chloride?
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Saerynide
Some water is oxidized, instead of chloride? |
Please, could you elaborate a bit?
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
At the anode, water can be oxidized into 4H+ and O2, instead of 2Cl- being oxidized into Cl2.
However, at the cathode, theres nothing else to reduce, except water, so there will be more H2 than Cl2.
If you electrolyse plain water, there will be more H2 than O2 as well, because for each molecule of H2, you get only 1/2 an O2.
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
maxke
Harmless

Posts: 7
Registered: 18-5-2004
Member Is Offline
Mood: No Mood
|
|
[Edited on 15-6-2004 by maxke]
|
|
|
maxke
Harmless

Posts: 7
Registered: 18-5-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by maxke
| Code: | 2 H+ + 2e- --> H2 0.00 |
| Code: | 2 Cl- --> Cl2 + 2e- -1.36 |
| Code: | Na --> Na+ + e- 2.71 |
| Code: | 2H2O(l) + 2e- --> H2(g) + 2 OH- -0.83 |
Very interesting effort.
But In the end you only have the four above things going on. I'have been a little out of service in my chemistry exercises ans such but you could
calculate the exact voltage required to stimulate emission of H2/O2 or H2/CL2 given a reservoir of NaCL/H20.
But then there are some energetic losses so it would be best to give a little extra voltage on top. With your 24v I'm sure you are producing CL2.
But ! If you want lots CL2 you need lots electrons. Needing a lot of electrons recquires high current, not a high voltage in my humble opinion.
Just make sure your voltage is high enough so that H2/O2 doesnt form.
| Code: | Even with a strong brine and 24V (lots of current), not much gas is produced. |
Have you measured the current ?
Preventing CL2 reaction of CL2 with H2O on the electrode position might be stopped introducing a pump, thus leaving the concentration of CL2 on water
level at a minimum.
But then again, it might be better to find a valve or some other device which does the same while doing it explosion INSENSITIVE.
In the end you are still working with explosive gasses.
But my respect goes out to you Tacho.
You have crafted that which I've been thinking about doing it in this or another way.
By the way; where would one acquire electrodes as you have ? |
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
| Quote: | Originally posted by maxke
If you want lots CL2 you need lots electrons. Needing a lot of electrons recquires high current, not a high voltage in my humble opinion.
|
More voltage = more current. Current is limitted by voltage, as i = v/r
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Saerynide,
Thank you very much. Yes, water electrolysing might be my problem.
The copper thing I mentioned goes as follows: The graphite anode was covered with copper that I removed by electrolysis, but maybe some copper was
left in the pores. As I begin to electrolyze the brine, the copper goes into the solution, reducing the output of H2. But now it’s being reduced at
the cathode, instead of Cl. My solution doesn’t look blue though. I’ll clean the electrodes and change the brine.
maxke,
I have not measured the current, but such a short path of strong brine must have little resistance and my power supply can provide more than 10A.
Besides, the setup gets warm and the power supply hums noticeably when I connect the wires.
| Quote: | | (snip) Preventing CL2 reaction of CL2 with H2O on the electrode (snip) |
What reaction?
About the electrodes: they are used by electric arc welders, I presume as a cutting tool. They are graphite rods with copper eletroplated on the
surface. I could not find them at the nearby (well supplied) hardware shop, I had to go to a shop specialized in soldering. They come in many
diameters and are quite cheap, about U$ 0,33 (0,25 euros) a piece.
|
|
|
maxke
Harmless

Posts: 7
Registered: 18-5-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Saerynide
| Quote: | Originally posted by maxke
If you want lots CL2 you need lots electrons. Needing a lot of electrons recquires high current, not a high voltage in my humble opinion.
|
More voltage = more current. Current is limitted by voltage, as i = v/r |
Ok, I agree , so basically you need a setup with low resistance because the powersource is one with a set voltage rating.
I had the concept of laser emission applied in this subject... so I might have some things mixed up.
Conclusion : there are 2 things you can do to increase current.
I= U/R
--> increase voltage
--> decrease resistance of the cell
Maybe overly saturating(NaCL) might give you better results ?? Distance between the two electrodes might also matter.
Tacho, I don't know if it is useful, but anyway : monitoring your current with a lowtech multimeter might also give an indication of the NaCl
concentration in the brine. A limitation however : the more H2 CL2 produced , the more NaOH is in solution , which is also conductive.
| Code: | Cl2 + 2 (Na+ + OH-) ––> (Na+ + ClO-) + (Na+ + Cl-) + H2O DrH°298 = - 103 kJ/mole. |
I was reffering to the formation of NaOCL. This is reffered to in my native language as "javel", natriumhypochlorite. This is used for
cleaning bathrooms and such. CL2 is basically absorbed in the watersalution containing the NaOH on the CL2 forming electrode.
Commercially "javel" is produced by bubbling CL2 through NaOH solution.
For finals : I'm sorry if I'm posting things that are obvious to you guys. I'm approaching this project as if I would try to do it
myself. I want to recreate it anyway. Due to lack of time (examinations) starting projects such as these are on hold for an undetermined time.
[Edited on 15-6-2004 by maxke]
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Yes, NaOCl may be an issue here.
I'll try this new design:
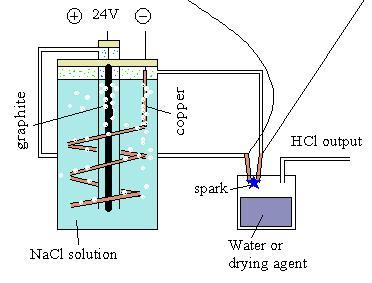
I believe this will circumvent the concentration of NaOH and improve Cl2 yield.
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
Nice drawing. You're going for a divided cell type effect, no? I'm afraid the only other way to aviod build up of OH- would be to cancel it
out, either with something simple like CO<sub>2</sub> (if this is acid enough), or possibly, if you feel like showing off, with anion
exchange resin.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
This idea is so crazy that I just <i>have</i> to try it myself!
Very impressive work, Tacho.
I'm thinking (or rather speculating): Couldn't a small, thin Pt wire inside the combustion chamber be used instead of the spark generator?
If memory serves me, it should catalyze the H2 + Cl2 --> 2HCl reaction....
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
USE ONLY SOFT PLASTIC FLASKS !!!!
I was lucky and frightened last night! While testing the new design, there was another explosion. Nothing happened, just a loud bang. But it
was inside the closed glass flask!
I could see the little fire running fast inside the silicone tube back to the generator and BANG! I believe it was the hydrogen/air mix.
I removed all copper from the anode using ferric chloride and used new brine. The new design produces lots of chlorine. The main problem now is
SAFETY.
axehandle,
A catalyst would be wonderful. Something more available than Pt would be really, really wonderful!
Any ideas? Would nickel powder react with HCl?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
| Quote: |
A catalyst would be wonderful. Something more available than Pt would be really, really wonderful!
|
Couldn't you remove the Pt film from a small, cheap type B, R or S thermocouple?
Note that I'm not entirely sure that Pt would initiate the reaction, so it could be an expensive failure...
How about this: I setup a small electrolysis, mix H2 and Cl2 in a test tube and then insert a Pt wire, then I'll tell you if it works or not?
This seems like such a good idea if it works that I'm very excited to try it out....
Edit: PS, be CAREFUL! Glass shards would make a horrible addition to one's eyes... *shudder*
Edit2: The Merck says that nickel is "slowly attacked by diluted hydrochloric acid".
Edit3: Another idea just struck me: If the device is powered by solar cells, the HCl will be FREE!!!
[Edited on 2004-6-16 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Note that Pt will ignite H2 in air, therefore if oxygen is present you could say bye-bye to your H2. I am not sure about the following but if I
remeber correctly platinum does react with chlorine gas, although I think it must be in somewhat 'high' concentrations. Well anyway, be
carefull about the catalytic ignition of H2.
Edit: Btw, sunlight was already mentioned, but following its disadvantage as a 'sure' catalyst why not use 'cheap' UV lamps. UV
triggers the breaking of the Cl-Cl bond into free radicals therefore intiating the reactions.
[Edited on 16-6-2004 by Esplosivo]
Theory guides, experiment decides.
|
|
|
| Pages:
1
2
3 |