| Pages:
1
2 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Glucose Nitroureide Tetranitrate
Here is an obscure energetic material for which I can find no additional information. It is a very interesting energetic material because of the
inexpensive precursors and relatively simple synthesis along with the additional interesting fact that a lead salt derived from the compound can act
as an initiator for the material which reportedly is sufficiently energetic and sufficiently stable for use in a compound detonator. It would also
seem likely that this nitrated aldose derivative would likely form binary or tertiary eutectics, gells or plastiques with nitrated sugars or nitrated
polyols.
US2969354 Glucose Nitroureide Tetranitrate
US2612497 Glucose Ureide from Glucose and Urea
Attachment: US2969354_ALDOHEXOSE_NITROUREIDE_TETRANITRATE.pdf (142kB)
This file has been downloaded 961 times
Attachment: US2612497_PROCESS_FOR_MANUFACTURE_OF_HEXOSE.pdf (135kB)
This file has been downloaded 881 times
[Edited on 25-6-2011 by Rosco Bodine]
|
|
|
gnitseretni
Hazard to Others
  
Posts: 282
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
Eh, kinda like the next ETN, only better, since you can make both a primary and secondary HE from it. I love it 
Great find Rosco 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yes indeed this is very interesting, and evidently GNT is obscure, as nothing comes up about it in searches. By virtue of it being acidic there are
numerous salts or combined salts which could have interesting properties. Lately guanyl azide is one of the energetic salt formers which has been
contemplated to form sensitive primary explosive salts, along with less sensitive secondary explosives from the isomerized form of guanyl azide that
is 5-aminotetrazole. Candidate
explosive acidic energetic compounds such as this Glucose Nitroureide Tetranitrate
might possibly form useful energetic salts with guanyl azide and 5-aminotetrazole,
with two of each being required for neutralization of the GNT. Perhaps would also be possible combined or bridged salts using one of each of the
guanyl azide
and 5-aminotetrazole with GNT. Azobisformamidine is dibasic and might also form an interesting salt with GNT. Such possibilities would suggest novel
experiments perhaps leading to several novel new explosive compounds.
Energetic GNT esters could also be possible. An interesting
blast from the past is this patent US2969354 from fifty years ago and it is obscure enough to definitely qualify as experimental. It looks like I sort
of started something with posting about ETN here a few years ago, which is another obscure and experimental compound. There aren't really any
"experts" on such little known and EXPERIMENTAL energetic materials, so danger is always present because of the unknowns. Please be safety conscious
and careful and thoughtful when any experiments are done particularly with obscure and experimental energetic materials.
[Edited on 26-6-2011 by Rosco Bodine]
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Very interesting... Well look at it this way... If you get caught pissing in sugar people are just going to think your crazy!!! LoL! Jk...
Looks like I found a use for my confectioners sugar leftover from rocketry. Not going to mess with the mess with the metal salts... You guys have fun.
The teranitrate looks promising though... Might have enough brisance to be useful.
Converted the patent into a easy to read synthesis... Please confirm these numbers are correct.
Hexose Ureides
1020g Sugar
340g Urea
30g H2SO4 (98%) + 30g H2O
1. Mix 102g Sugar + 85g H2O at 60C
2. Add 340g Urea.
3. Solution stabalized at 60C.
4. Subsequently 918g Sugar is then added in 102g portions.
5. The H2SO4 Solution is slowly added over an hour maintaining 60C.
The mixture is then reacted at 60C for eight hours.
Edit: Fixed synthesis
[Edited on 26-6-2011 by freedompyro]
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Either this reaction needs (16.5mL)30g H2SO4 or it needs (148.5mL) 270g... Can't figure out this guys wording!
[Edited on 26-6-2011 by freedompyro]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Condensation product with formaldehyde should be a reasonably dense polymer resin.
.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 282
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
It needs 16.5ml of 98% H2SO4.
BTW, how did you get at 85g water in step 1? They used 300ml water with the glucose and 550ml water when they used cane sugar. So divided by ten you'd
need 30ml water if using glucose and 55ml water if using cane sugar. So how did you came up with 85ml?
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gnitseretni  | It needs 16.5ml of 98% H2SO4.
BTW, how did you get at 85g water in step 1? They used 300ml water with the glucose and 550ml water when they used cane sugar. So divided by ten you'd
need 30ml water if using glucose and 55ml water if using cane sugar. So how did you came up with 85ml? |
Sorry man... Read the patent again. Your wrong. I have it printed out... Here is what it says exactly.
", while the quantity of water was increased by 550 grams in order to bring about the hydrolysis."
"increased by" are the key words.  It doesn't say "increased to" It doesn't say "increased to"
Now we just need someone to actually make the stuff and verify the yield... The last thing I am confused about is how to deacidify it... If it's water
soluable thats going to be a pain in the neck. If you use sodium bicarbonate won't the sodium stay in the water through the evaporation? Meh...
Oh wait, I have barium carbonate on me... Doh!
[Edited on 26-6-2011 by freedompyro]
|
|
|
gnitseretni
Hazard to Others
  
Posts: 282
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
@freedompyro: Yes, you're right. My bad!
I found a paper that gives a different way to make glucose ureide. I don't care if that method is better or not because the process takes much longer
than the one in the patent Rosco provided. However, in it are mentioned some solubilities of glucose ureide urea, not glucose ureide, but
they said the solubilities "closely parallel those of glucose ureide".
| Code: | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251697/pdf/biochemj01152-0221.pdf |
Readily soluble in water.
Very sparingly soluble in ethyl, methyl alcohol.
Practically insoluble in acetone, and quite insoluble in ether, chloroform and non-hydroxy solvents.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
diglucose ureide patent US2967859
Please elucidate, elaborate. I have wondered about this myself. The glucose ureide could make a cheap and useful binder and fuel for pyrotechnics.
Also a 10% excess of the theoretical requirement of urea is used in the described process for producing the glucose ureide, so the residual liquor
from the reaction would contain the unreacted urea.
There are patents noting the usefulness of urea-formaldehyde resin as a very good sensitizer and fuel for ammonium nitrate. And urea is a known
sensitizer fuel itself
as well as good for lowering the melting point of AN. It would seem likely then that the entire reaction mixture from
the production of the glucose ureide could be useful as a
fuel sensitizer for AN as well as having potential for further
reaction involving polymerization via reaction with an added
formaldehyde component. A melt cast and polymer bindered
AN based composition having useful properties could be the
ultimate result.
Attached is US2967859 for Diglucose Ureide which may also lead to an interesting energetic material upon nitration.
Attached also is the article linked above by gnitseretni which describes an adduct of glucose ureide with urea. In the first patent a 10% excess of
the theoretical requirement of urea is used beyond what is needed to form glucose ureide, so the excess urea must react further to form the adduct of
urea with the glucose ureide already formed ....and the adduct probably remains in solution in the mother liquor from which the main product the
glucose ureide crystallizes.
Attachment: US2967859_PROCESS_FOR_DIGLUCOSE_UREIDE.pdf (166kB)
This file has been downloaded 755 times
Attachment: Glucose Ureide Urea adduct biochemj01152-0221.pdf (490kB)
This file has been downloaded 754 times
With regards to monoglucose ureide here is additional information

Name: Glucosylurea
Name english (2nd lang): Glucosylurea
Molecular Formula: C7H14N2O6
IUPAC: [(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]urea
CAS: 5962-14-1
Molecular Weight: 222.196 g/mol
Exact Mass: 222.085 g/mol
Monoisotopic Mass: 222.085 g/mol
SYNONYMS
Glucosylurea
Glucose ureide
Glucosyl urea
Urea, glucosyl-
Urea, D-glucopyranosyl-
AIDS022743
AIDS-022743
CID151391
5962-14-1
Elemental composition: C7H14N2O6
Element Symbol Atomic Mass # of Atoms Mass %
Carbon C 12.01078 7 37.84%
Hydrogen H 1.007947 14 6.35%
Nitrogen N 14.00672 2 12.61%
Oxygen O 15.99943 6 43.20%
[Edited on 26-6-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I don't really need to plan it because I already have everything on hand.
I would like to do the synthesis when time and opportunity permits.
But that may not happen for awhile, so I am basically sharing what I found
at this point for anybody else who may get to it before I do.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 282
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
Yeah, I'm a bit short on time as well. Making the GNT is no problem, it's the ureide that's the problem due to the long reaction time. But the
precursors are cheap, so when you do find time to perform the synth, it makes sense to make enough so you don't have to do it again anytime soon.
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gnitseretni  |
Readily soluble in water.
Very sparingly soluble in ethyl, methyl alcohol.
Practically insoluble in acetone, and quite insoluble in ether, chloroform and non-hydroxy solvents. |
I don't really think we need to worry about purifying it. Just deacidification.
We can purify it properly after it's nitrated.  Probably a LOT easier... Probably a LOT easier...
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
26-6-2011 at 15:17 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by holmes1880  | | Matt, you should stop moving my posts. I have a legitimate concern that Bodine is refusing to synth shit he digs up, but tries to sell others on
making it. |
@holmes1880 I am not selling anything. I am sharing some interesting information I found while looking for something else. Your concern here may seem
legitimate to you, but like your concern about some other things your concern lacks validity. I am not "refusing" to synthesize anything. I said I
would like to experiment with this when I have time. If you don't accept that or don't like that, I really don't care. If you can't contribute useful
information to a thread then you should refrain from posting anything at all until you have something useful to contribute. Avoid being a compulsive
poster. Nobody likes it, and it will likely get you banned.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Holmes, I wish everyone could and would try out every interesting idea that they come across. But I know that's not practical. Nobody here is my
employee and I can't demand that they do work. I would rather read about an interesting untried idea than require only completed research be posted
here. As an example, people had started posting about the patented production of potassium metal from KOH and Mg in solution years before Pok got it
to work. There was a lot of talk and not a lot of action, but without the talk here and on related forums I don't know if Pok would have come across
the idea when he did.
PGP Key and corresponding e-mail address
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Or calcium cyanamide from cyanurate. Rosco's idea but I was the first to try it, and it worked.
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
What we need now is for someone to get a RE/Brisance estimation done by cross comparing the performance of GNT with well researched HE's. It will
probably be a couple weeks until I can do full testing on GNT. Probably will just cross compare it with Nitrourea. If it outperforms... Continued
testing.
My gut hunch is that Glucose Nitroureide Tetranitrate will give some respectable performance with a greater than 1.0 RE and detonation speed greater
than 6500m/s. NitroUrea detonates at greater than 5400m's when compressed to around 1.1 g/cm3 with a RE of 0.94 according to my documents.
I am going to be very disappointed if this doesn't have substantially higher performance than NitroUrea. First to do testing please tell us your
results. 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Glucose Pentanitrate has nitrogen content of 17.29%
PETN has nitrogen content of 17.71%
Glucose Nitroureide Tetranitrate has nitrogen content of 21.9%
Nitrourea NO2-NH-CO-NH2 , CH3N3O3 has nitrogen content about 40%``
Stability for the Glucose Nitroureide Tetranitrate is reportedly okay at normal storage temperatures. I am guessing, but I would expect density
around 1.7
and an RE around 120%.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Rosco Bodine
" Please elucidate, elaborate. I have wondered about this myself."
In regard to polymer formation I have no insight beyond the observation that
as stated in US 2512497
" In general the process according to my invention aims at the preparation
of such derivatives of hexoses and the nitrogen compounds referred to
as do not polycondense in vitro to insoluble macromolecules in the presence
of formaldehyde and an acid catalyst, as for instance urea itself would do
within a few moments with formaldehyde;"
However as stated in US 2969354
" The esters so obtained are acidic in nature and readily form metallic salts."
Whether the non reactive nature to polycondensation of the ureide precursor
carries onto the tetranitro product is unclear to me. The material being acidic
however does imply it must react with aldehydes generally, though not perhaps
with the amine functional group. Urea formaldehyde comparatively is comprised
of a mix of different products dependent on reaction conditions.
Attachment: Urea Formaldehyde.pdf (142kB)
This file has been downloaded 1485 times
___________________________________________________
I have investigated comparative products obtained from cyanuric acid
Cyanuric acid exists almost exclusively in the tri-keto form in acid solution.
Isocyanuric or tri-keto structure exists in acidic solutions, below pH 6.
The di-keto structure predominates in the range from pH 7 to 10.5,
and the mono-keto structure above pH 11. In very strongly basic solution,
it is probably almost completely enolized (OH).
Cyanuric acid is only slightly soluble in water, 0.125%, or about
1 part in 800 parts of water at room temperature and 4% in boiling
water. It is even less soluble in alcohol.
In reactions of 1 mol of isocyanuric acid with 3 mol of formaldehyde one obtains
1,3,5-tris(hydroxymethyl) isocyanurate (THMIC)
also known as 1,3,5-tris(hydroxymethyl)-s-triazine-2,4,6-trione
and Tris(hydroxymethyl) isocyanurate or Trimethylol isocyanurate
1,3,5-Trimethylol-1,3,5-Triazine-2,4,6-Trione
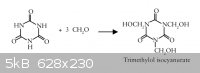
See this excerpt => Attachment: Trimethylol Isocyanurate.pdf (141kB)
This file has been downloaded 593 times
from Chemistry and Technology of Polyols for Polyurethane http://ifile.it/kthzvaw
and British patent => Attachment: Trimethylol Cyanurate GB420525.pdf (542kB)
This file has been downloaded 613 times
From Kirk Othmer
Cyanuric acid readily dissolves in aqueous formaldehyde forming tris(hydroxymethyl)isocyanurate (THMIC) CAS 1047-40-6
which can be isolated by evaporation. [11] The Chemistry of Heterocyclic compounds vol 13 , 17-146
here => http://ifile.it/r3l7n8b
on Pg 48
Resinous condensation products are prepared by heating cyanuric acid
with formaldehyde With [148] or without [149] a diluent ; a clear resin
forms. Acid condensation agents such as hydrogen chloride may be
used to promote the reaction.
My note : this should be nearly quantitative yield, and the trinitrate readily made from it.
My initial interest waned as the projection of performance is disappointing.
The Trazine Trione skeleton has a high heat of formation which evidently degrades
the heat of explosion of what is essentially three attached Methyl Nitrates.
___________________________________________________
A variation on this theme requires glyoxal which is relatively expensive although
required starting materials are available over the counter. The tetranitrate of
compound [ 20 ] is oxygen balanced and should compare favorably with PETN.
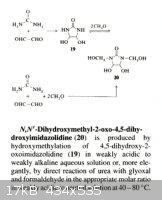
____________________________________________________
Finally the condensation product with hydrazine is a base which can form
nitrate or perchlorate salts.
2,4,6-Trihydrazone-1,3,5(1H,3H,5H)-Triazine
1,3,5-Triazine, 2,4,6-trihydrazinyl
2,4,6-Trihydrazino-1,3,5-triazine
Trihydrazino-s-triazine
Cyanuric trihydrazide CAS 10105-42-7
http://webbook.nist.gov/cgi/cbook.cgi?ID=C10105427&Units...
http://cdb.ics.uci.edu/cgibin/ChemicalDetailWeb.psp?chemical...
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6627...
.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The first variation which I thought might be possible is a peroxide at the keto oxygen of the (tetranitro ester substituted) nitrourea.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Polydextrose nitroureide polynitrate polymer possible??
@franklyn
Generally on the topic of polymerization, not with regards to the GNT, but with regards to the dextrose itself which is a precursor reacted with urea
to form the intermediate for nitration, the question is raised whether or not urea would also react with polydextrose to form a polydextrose ureide
which could then be nitrated to a polydextrose nitroureide polynitrate ? It is unknown whether or not the polymerized dextrose would react with urea
to form a ureide as does the monomeric dextrose. If it does react to form a nitratable intermediate then the resulting nitroureide would contain less
of the nitrourea moiety and would be expected to be more stable yet as well as being more mildly acidic.
There is nothing I have found in the literature about this hypothetical compound derived from polydextrose and urea.
Polydextrose has been identified as a useful material for nitration and the nitrated product has good energy and stability. See US2495868 attached.
The polydextrose precursor is easily made by a number of methods involving heating the dextrose with a dry catalyst such as boric acid which appears
to have a similar dehydrating and polymerizing effect upon dextrose as
is involved in the formation of dextrins from starch.
See US2375564 attached and US2400423 attached and
US2436967 attached
Attachment: US2495868_Nitrated Dextrose Polymer.pdf (154kB)
This file has been downloaded 671 times
Attachment: US2375564_Dextrose Polymerization Boric Acid Catalyst.pdf (819kB)
This file has been downloaded 702 times
Attachment: US2400423_POLYMERIZATION_OF_MALTOSE.pdf (487kB)
This file has been downloaded 880 times
Attachment: US2436967_POLYMERIZATION_OF_SUGARS.pdf (413kB)
This file has been downloaded 2532 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
A neat fibre such as nitocellulose would be desirable , a pasty product like caramel
would not be , hampering it's solubility and processing with acid. The field as you
observed has not been investigated and appears is quite extensive. Linear or ring
structured sugars provide varing physical attributes and the eventual oxygen balance
will determine what performance can be expected.
Carbamide derivatives of Sugars - Abstract
http://books.google.com/books?id=yfI4AAAAMAAJ&lpg=PA258&...
Carbohydrate Based Condensation Resin - Patent
www.taeus.com/patent_attorney_tracker/profile.php?sub=downlo...
Glycosyl Ureides , Irving Goodman , pages 215 - 236
Advances in Carbohydrate Chemistry & Biochemistry Vol 13
http://books.google.com/books?id=NDnNCOG6RWcC&lpg=PA215&...
Nitro Sugars , H. H. Baer , pages, 67 - 138
Advances in Carbohydrate Chemistry & Biochemistry Vol 24
http://ifile.it/i2oyqw8
http://en.wikipedia.org/wiki/Caramel
http://www.museumstuff.com/learn/topics/caramel::sub::Chemis...

Mechanism for the formation of sugars from formaldehyde
http://ia700503.us.archive.org/15/items/nasa_techdoc_1968001...
The Technology of Sugar
http://ia600407.us.archive.org/33/items/cu31924003619909/cu3...
P.S.
I had cited this in another post
Low Melting Sugar – Urea - Salt Mixtures as Solvents for Organic Reactions
www.rsc.org/suppdata/CC/b4/b414515a/b414515a.pdf
Some are liquid at less than 70 ºC , indicating condensation to a ureide
is not clearcut and higher reaction temperature is needed.
.
[Edited on 15-7-2011 by franklyn]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I believe these dextrose polymers are similar to an oligosaccharide intermediate between sugar and dextrin, an indigestible soluble fiber type of
material, but shorter chained, as is dextrin related to microcrystalline cellulose, but the soluble "fiber" characterization is something of a
misnomer because the character of these lower polymers is more distinctly crystalline or granular than fibrous. Likewise the nitrated forms should or
would be expected at least some them to inherit the gritty consistency of the precursor material. Compare to fructo-oligosaccharide and inulin.
[Edited on 18-7-2011 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Rosco Bodine  | Yes indeed this is very interesting, and evidently GNT is obscure, as nothing comes up about it in searches. By virtue of it being acidic there are
numerous salts or combined salts which could have interesting properties. Lately guanyl azide is one of the energetic salt formers which has been
contemplated to form sensitive primary explosive salts, along with less sensitive secondary explosives from the isomerized form of guanyl azide that
is 5-aminotetrazole. Candidate
explosive acidic energetic compounds such as this Glucose Nitroureide Tetranitrate
might possibly form useful energetic salts with guanyl azide and 5-aminotetrazole,
with two of each being required for neutralization of the GNT. Perhaps would also be possible combined or bridged salts using one of each of the
guanyl azide
and 5-aminotetrazole with GNT. Azobisformamidine is dibasic and might also form an interesting salt with GNT. Such possibilities would suggest novel
experiments perhaps leading to several novel new explosive compounds.
Energetic GNT esters could also be possible. An interesting
blast from the past is this patent US2969354 from fifty years ago and it is obscure enough to definitely qualify as experimental. It looks like I sort
of started something with posting about ETN here a few years ago, which is another obscure and experimental compound. There aren't really any
"experts" on such little known and EXPERIMENTAL energetic materials, so danger is always present because of the unknowns. Please be safety conscious
and careful and thoughtful when any experiments are done particularly with obscure and experimental energetic materials.
[Edited on 26-6-2011 by Rosco Bodine] |
An error has been discovered in my post above that the
Glucose Nitroureide Tetranitrate has a dibasic character for neutralization which I should correct now realizing from checking the molecular weight
and nitrogen analysis for the sodium salt reported indicates the Glucose Nitroureide Tetranitrate is neutralized by 1 sodium rather than two.
The molecular weight for
Glucose Nitroureide Tetranitrate is 447.15 corresponding with the patents reported theoretical Nitrogen content of 21.9%
A monosodium salt of Glucose Nitroureide Tetranitrate
would have a molecular weight of 469.14 and a Nitrogen content of 20.88% which is more in agreement with the patents reported theoretical Nitrogen
content of 20.8%
than would be the theoretical Nitrogen content of a disodium salt which would be 19.95% based upon a molecular weight
of a disodium salt of 491.15
Therefore a bit of computational analysis shows that the
Glucose Nitroureide Tetranitrate is a monobasic acid.
Further computational analysis shows that the nitration accomplished by using the 7 parts of 50/50 nitration acids
as was used for preparation of the Glucose Nitroureide Tetranitrate precursor for the lead salt increased the yield for the nitration from the 60%
yield gotten by 10 parts used in the first example, to a yield of 76.75% of Glucose Nitroureide Tetranitrate, based upon the reported yield of the
lead salt of 19 grams, the molecular weight for the lead salt calculated to be 1099.5 by one mole Pb and 2 moles Glucose Nitroureide Tetranitrate
(less two hydrogens)
The nitrogen content for the lead salt should be 17.8%
[Edited on 8-9-2011 by Rosco Bodine]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
It's a damn interesting patent and certainly (superficially) commercially viable. I checked a bit and except for some material Franklyn already found
I couldn't find much. The ISEE's text had nothing, etc. I wonder why this was not commercially popular? Perhaps there are shelf life limitations.....
It seems odd that such a thing that demands so little did not have a commercial following. It appears Atlas Powder Company bought or subsidized the
patent in '61 yet so little is known about it.
|
|
|
| Pages:
1
2 |