| Pages:
1
2
3 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
That's surprising really, because of the high cost of nitromethane. Isn't the bisulfite/nitrite method for hydroxylamine production way more
economical?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Well, in Ullmann's Encyclopedia of Industrial Chemistry it says that "This process has been used only to a limited
extent because of its cost and the limited availability
of raw materials." about the NM/acid process.
The Raschig process is more important as is the catalytic hydrogenation of NO or nitrates.
But since I can buy NM and not nitrite, the acid/NM method is the one I'm giong with.
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Thanks for translation, alternatives, experiances!
| Quote: | Originally posted by chemoleo
Also, it mentions that the hydroxylamine HCl can be reacted with cyanamide to form oxyguanidin hydrochloride, CN3H5O:HCl. What precisely is this?
Anyone knows?
|
H2N-C(=NH)-O-NH2 . HCl?
There is mention of (NH2O)2C=NH here,
http://ww1.ft100.net/~64671/xfiles.ft100.net/images/oxylamin...
forget what the actual journal ref this come from, I have so much of this crap on my computer, in german ... again 
| Quote: | Originally posted by Microtek
diamino furazan can be made from condensation of glyoxal and hydroxylamine to diaminoglyoxime |
Were you able to do that in good yield in one step? See pg 377 of preceedings <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=5393">here</a>, supposedly you can go all the way to diaminofurazan in one step
from glyoxal/NH2OH.HCl/NaOH + "other", but specifics arn't mentioned.
I've isolated glyoxime, then diaminoglyoxime from that, but only in ~20% from glyoxal -> DAG.
[Edited on 25-2-2006 by Axt]
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
No, I haven't actually tried that yet as I've only just gotten the hydroxylamine production in place. I plan to follow the modification proposed in
"Furazan-based Energetic Ingredients" which I think I got from you at the Crucible.
The modification involves heating an excess of free hydroxylamine to 90 C and then dripping in glyoxal soln while maintaining the mix at 90 C.
According to the article I mentioned, yield suffers if temp rises above 90 C which can be difficult to control if mixing the ingredients first and
then heating. This should give diaminoglyoxime directly in 64 % yield ( at the 1000 g scale ).
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
More uses of hydroxylamine
I had a look at Axt's article from above. It contains some interesting chemistry, some that even seems doable!
For instance, potassium hydroxylamine disulphate (KO-N(SO3K)2), which is made as described above from sulfite and nitrite, is a useful reagent for the
reaction with alkylating agents, for instance dimethylsulphate or, more realistically, halogeno-alkanes.
Alpha-methyl hydroxylamine, CH3-O-NH2
Basic potassium hydroxylamine disulphonate (dissolved in 6% KOH) is mixed with the same molar quantity of dimethylsulphate or methyliodide. The latter
disappears within one day, while DMS reacts much faster while heating up. This can be boiled down, and alpha methyl hydroxylamine dipotassium
disulphonate CH3-O-N(SO3K)2 crystallised in good yields, which can be recrystallised in slightly ammonia-containing H2O.
To split the N-SO3 bonds, the above salt is boiled in H2SO4, under reflux. I'm not sure if reflux is necessary, but probably topping up with H2O now
and then would be good. The end of the reaction is achieved after several hours. (The end is recognised by taking a small aliquot of the solution,
adding BaCl2/OH2 to remove extra H2SO4, and nitrite is added, which will react with the hydroxylamine. If H2SO4 is released, then addition of further
BaCl2/OH2 will result in further precipitation, which indicates unreacted sulphonic acid. Thus more boiling is required).
Once the reaction is finished, the CH3ONH2:H2SO4 salts are extracted with ethanol, seemingly recrystallisation in ethanol can separate the less
soluble neutral salt ((CH3ONH2)2:H2SO4) from the more soluble acidic salt (CH3ONH2:H2SO4).
They summarise this to make this reaction as fast as possible:
1) methylate the potassium hydroxylamine disulphonate in basic solution, as described above.
2) Boil this with H2SO4 or HCl to dryness, while the former is prob better (no HCl fumes), and test for completion of the reaction.
3) Add NaOH to a stoichiometric excess (cooling), and boil and distill. Free methyl hydroxylamine base comes over with the water, and is directely
either collected in acid, or simply in a cooled container. Yields, wrt the starting compound, is 80%
Interestingly, the methyl hydroxylamine nitrate, explodes with great force when heated to 300 deg C. The nitrate is well-soluble in absolute ethanol,
which may provide a purification technique. Maybe, Axt, that's something you want to try!!!! 
Alpha, alpha ethylene dihydroxylamine, H2N-O-CH2-CH2-O-NH2)
Again, energetic compounds of this hydroxylamine can be easily seen 
Here, 1,2 dibromo ethane is required, Br-CH2-CH2-Br. The sulphonate salt from above is reacted with it, in a 2:1 proportion, as described with the
methyliodide above. This forms the tetrasulphonate, similar to the reaction above, which precipitates/crystallises from solution due to its low
solubility:
(KSO3)2N-O-CH2-CH2-O-N(SO3K)2
This is digested with H2SO4, under boiling and testing for the end of the reaction as described above. The free based distills at 203 deg C.
Next to making the nitrate salts, I wonder how good a substrate these hydroxylamines are for peroxidation...? Similar to the 'amine peroxide' thread.
Other than alkylhalides, the initial hydroxylamine sulphonate also reacts with cyanogene bromide or iodide. Sadly this is not an easy substnace, nor
safe. This then forms the imine sulphonate, (KSO3-N-O)2C=NH. However, this does not form the (NH2O)2C=NH, which Axt mentioned, even though boiling
this with acid should theoretically get this.
Altogether, I think this may open up potential for a whole series of compounds, given the right starting compounds (alkylhalides).
Who's going to make some methyl-hydroxylamine nitrate? 
[Edited on 28-2-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
I'm attaching an article which supposedly gives the synthesis of NH2OH.HCl by refluxing HCl with nitromethane.
<a href="http://xfiles.ft100.net/images/nm-hcl-hydroxylamine.pdf">Journal f. prak. Chemie, Vol 21, 1880, page 129</a>
Can a german speaker please look at that and give the specifics of the reaction (quantities, reflux time, extraction etc.). If its in there ...
somewhere.
More random NH2OH articles I have:
http://scibooks.ft100.net/images/hydroxylamine.zip |
Those links are broken.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Try to secure them here........solo
http://rapidshare.de/files/14438117/nm-hcl-hydroxylamine.pdf...
http://rapidshare.de/files/14438496/hydroxylamine.zip.html
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
No. The links are alright.
I could download both files.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Thanks for the renewed links, Solo.
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
For some reason the links seem to work for some but not others.
I remember where that "oxylamine" article come from, it was referenced to in PATR2700 under the entry ethylenedioxyamine. Making it Ber 53, 1489
(1920).
PATR2700 gives no actual figues but a few properties, the dinitrate detonates when heated over its melting point, and the diperchlorate is responsible
for killing the two chemists working with it  they refer to it as a "sensitive
material". they refer to it as a "sensitive
material".
Apart from the salts and possible peroxides, complexes are likely and chloramines may be possible as well. It would be interesting to try the same
peroxide/complex/salts/chloramine with diaminofurazan as is possible with the alkyl amines.
The O-methylhydroxylamine or methyloxyamine is patented as rocket fuel as replacement for hydrazine US3117415, though the only sythesis reported is
that translated by Chemoleo.
|
|
|
gilaneh
Harmless

Posts: 1
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
i have a question. do you know anything about role of pyruvic acid in biosynthesis of l-PAC?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Yes, I know it's not clearly on-topic.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Wikipedia says nitrATE and bisulfite will form hydroxylamine. Im pretty sure NO3- will reduce to NO2- and then will form hydroxylamido-N,N-disulfate
anion. Heat for 1 hr at 100oC in acid to get hydroxylamine salt.
Sounds easy and I might try it. Any suggestions on what I should expect? For example, how will I know when the potassium hydroxylamido-N,N-disulfate
has formed?
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Does bisulfite reduce potassium nitrate to nitrite?
I mixed some nitrate with Iron Out(sodium dithionite and sodium bisulfite) and some HCl in ice water. And nothing seemed to happen except SO2 gas
coming off. I thought the nitrate would oxidize the the SO2 quickly.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Don't trust Wikipedia on such things. Bisulphites are in my opinion unable to reduce the nitrate anion. In acidic enough conditions HNO3 should
oxidize H2SO3 (or SO2). However this would produce NO2 and ultimately NO, but not hydroxylamine.
I think some Wiki author made a very common mistake of translating the old term "hydrosulfite" to bisulphite when it actually means dithionite. I
assume this since sodium dithionite might perhaps be an appropriate reagent for the nitrate->hydroxylamine reduction in basic enough media, but I'm
not familiar with inorganic chemistry so rather check Gmelin for the reference by yourself.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
I actually wrote down the equation for the reaction of nitrate with bisulfite and it turnes out that H+ does not play any role! So it would only react
extremely slow in aqueous but it would work when the two are heated together (as I just read in the ionic nitrites thread).
|
|
|
overunity33
Harmless

Posts: 35
Registered: 3-1-2010
Member Is Offline
Mood: No Mood
|
|
Hydroxylamine HCL synthesis from nitromethane+acetic acid+HCL
I found this description somewhere :
| Quote: |
400 mls nitromethane, 600 mls Acetic acid and 600 mls HCL are heated to just below reflux and held there for 2 hours then refluxed for 12 hours. The
solution is reduced to half gone the cooled in fridge overnight and filtered.
|
I don't understand the mechanism behind the reaction, can anyone explain it? Yields are reported to be 100%+ somehow. What can the resulting product
be washed/recrystallized with to increase purity? I assume this reaction utilizes glacial acetic acid and muratic acid, anyone have insight?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
"I found this description somewhere" does not say where you found it! 
The only way to know more about the mechanism would be to check the original paper where the reaction was described. Sometimes the authors propose the
mechanism and give supporting evidence or further reference. So why don't you check the literature? Two references where already given in our hydroxylamine thread, so half of the work is already done. All there is for you is a trip to the library.
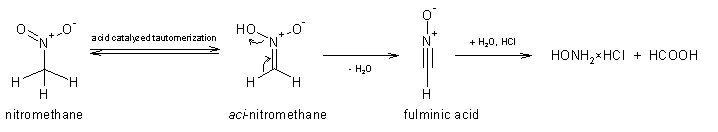
The interpretation of the reaction by using the "likely mechanism" approach as though to students would give a tautomerization-elimination-hydrolysis
route. The mechanism for the tautomerization of nitromethane is described in many schoolbooks, the mechanism of the elimination might or might not
first involve the protonation of aci-nitromethane, while the hydrolysis of fulminic acid is rapid at such conditions and is well known since
the year 1882 (J. Chem. Educ., 77, 851-857). I'm not going to waste time drawing such a mechanism proposal, but in short version and skipping
a whole lot of intermediates and all the arrow pushing:
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
overunity33
Harmless

Posts: 35
Registered: 3-1-2010
Member Is Offline
Mood: No Mood
|
|
I wrote about this in another thread because I couldn't find this one. I found a method somewhere else but I could not find literature or any other
type of reference.
(double post removed)
Edit by Nicodem: I merged your thread with this one. Next time do not double post, particularly not if you have already been
receiving answers to your original post! And it is "HCl" as for hydrochloric acid, and not "HCL" as you keep mistaking.
[Edited on 27/1/2010 by Nicodem]
|
|
|
Nicodem
|
Threads Merged
27-1-2010 at 01:45 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
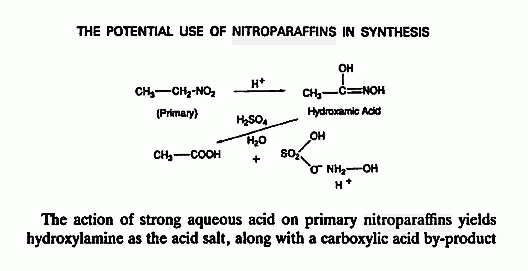
Hydroxylamine has been considered as a monopropellant a possibility is the
borane salt , 3 HONH2•BH3 => B2O3 + BN + N2 + 9 H2
Related threads _
This reference on Hydroxylamine produced by reduction of HNO3 by electrolysis
http://www.sciencemadness.org/talk/viewthread.php?tid=1716#p...
is complimented by the detailed mention in the lower image posted below specifically
citing the method outlined on page 32 of The Manufacture of Chemicals by Electrolysis
from here _ http://www.sciencemadness.org/talk/viewthread.php?tid=13871#...
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Many thanks to kmno4 for alerting that Journal of Energetic Materials is for now freely accessible.
http://www.sciencemadness.org/talk/viewthread.php?tid=3724&a...
These interesting amine bases and their salts related by chemoleo here
http://www.sciencemadness.org/talk/viewthread.php?tid=1928&a...
methyl hydroxylamine nitrate, and ethylene dihydroxylamine, are not " hydroxyl " amines
once that has been replaced by an organic functional group. The nomenclature is then
Oxyamine. These analogs of methylene diamine and ethylene diamine contain oxygen bridging
the carbon and nitrogen. The adduct of Methylene (bis)Oxyamine CH2(-O-NH2)2 with
Nitramine or Dinitrourea is unresearched. CH2(-O-NH2)2 •2 H2NNO2 => CO + 5 H2O + 3 N2
CH2(-O-NH2)2 • (HNNO2)2CO => CO2 + CO + 4 H2O + 3 N2
Synthesis and characterization of Methylene (bis)Oxyamine CH2(-O-NH2)2 salts
http://www.informaworld.com/smpp/ftinterface~content=a752776...
Synthesis and Characterization of Energetic 1,2-(bis)Oxyamino Ethane Salts
http://onlinelibrary.wiley.com/doi/10.1002/prep.200600027/ab...
High Density Energetic Mono- or (bis)Oxy-5-Nitro imino tetrazoles.pdf ( copy both lines below together )
http://www.chtf.stuba.sk/~szolcsanyi/education/files/Chemia%20heterocyklickych%20zlucenin/Predn%E1%9Aka%203/Doplnkov%E9%20%9Atudijn%E9%20materi
%E1ly/High-Density%20Energetic%20Mono-%20or%20Bis(Oxy)-5-Nitroiminotetrazoles.pdf
References from the bibliograpphy 15, 16 , 17
[15]
a) C. S. McDowell, M.W. Barnes, U. S. Patent 3709920, 1973;
b) C. S. McDowell, C. Merrill, U. S. Patent 3714200, 1973;
c) C. S. McDowell, C. Merrill, U. S. Patent 3714199, 1973;
d) Russ. J. Org. Chem. 2004, 40, 124 – 126
D. V. Davydov, I. P. Beletskaya,
http://www.springerlink.com/content/r260w7181k546243
Available from Madhatter's archive
[16] ( these are the first 2 links cited above )
a) J. Energ. Mater. 2001, 19, 277 – 303;
K. Tollison, G. Drake, T. Hawkins, A. Brand, M. McKay, I.Ismail, C. Merrill,
b) Propellants Explos. Pyrotech. 2006, 31, 196 – 204.
T. Hawkins, L. Hall, K. Tollison, A. Brand, M. McKay,
( had been posted as a download from rapidshare )
[17] Synthesis of 12: ( 1,2-(bis)Oxyamino Ethane )
J. Org. Chem. 1984, 49, 4487 – 4494.
D.W. Dixon, R. H. Weiss,
related thread
http://www.sciencemadness.org/talk/viewthread.php?tid=13174
.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
http://www.erowid.org/archive/rhodium/chemistry/hydroxylamin...
Check this method, maybe it works. I think I saw that method on illumina-chemie.de also, but it gave very low yield, maybe they did not do it right.
I could try this method on test tube scale, just I need to substitute potassium salts for sodium. Would that work then? I only have potassium
metabisulfite.
By the way, I read on wikipedia that it is explosive, is it? I dont want to prepare compounds that will blow up me.
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
Quote: Originally posted by Organikum  |
Even more funny is that phenylacetylcarbinol is WRONG as this describes an isomer - but as Neuberg and Hirsch who discovered the stuff made this
mistake in their first publications (corrected it lateron) the name is still used.
|
weird
[Edited on 13-8-2011 by MeSynth]
|
|
|
poopyMcgee
Harmless

Posts: 1
Registered: 2-9-2011
Member Is Offline
Mood: No Mood
|
|
Thought i would chime in!
Reagents
150 ml 36% HCL
150 ml Glacial Acetic Acid (LG)
100 ml Nitromethane (Hobby Grade, apparently 99%)
Method
HCL, GAA and NM added to 1L flask and manually mixed. Mixture was heated, without mixing, to 60C and held for 30 minutes and then gradually increased
to 90C and held there for 2 hours. Mixture took on yellow translucent appearance. Gentle reflux was achieved within 1L flask, no vapor was entering
condenser. After 2 hours temperature was increased to 103C to achieve reflux and held for a 12 hour period. Mixture was cooled and left to stand for
24 hours. A white crystalline mass was achieved, flask was subsequently decanted, crystals agitated, removed and dried with a weight of 25 g. Decanted
liquid was then reduced to half it volume, cooled and again decanted another 48g of crystalline material was recovered.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: | Preparation of Hydroxylamine hydrochloride by reduction of nitrite, using sulfur dioxide
40g potassium nitrite (KNO2) and 50g potassium acetate (CH3COOK) are dissolved in 100ml ice water. 750g finely crushed ice is added. Into this
solution a stream of sulfur dioxide (SO2) is bubbled until the solution smells of SO2. The temperature must be kept below 0°C through the whole
reaction. The salt of K2[HON(SO3)2] separates and is filtered off and washed with ice water. The salt is dissolved in 500ml 0.5 M HCl and boiled for
two hours. Still boiling a solution of Barium chloride (BaCl2) is added as long as barium sulfate (BaSO4) precipitates ( CaCl2 may possibly work
instead). The BaSO4 is filtered off and the clear filtrate is evaporated to dryness. The residue consists of Potassium Chloride (KCl) and NH2OH*HCl.
Anhydrous ethanol(EtOH) is used for extraction of the Hydroxylammonium chloride, the KCl remains undissolved. The EtOH is evaporated on a water bath
and the product can be recrystallised from water (mp 151°C).
Mix cold saturated solutions containing one molecular proportion of sodium nitrate, and two molecular proportions of acid sodium sulphite, and then
adding a saturated solution of potassium chloride to the mixture. After standing for a day, hydroxylamine potassium disulphonate crystallizes out.
This is boiled for some hours with water and the solution cooled, when potassium sulphate separates first, and then hydroxylamine sulphate.
|
Free hydroxylamine is both unstable and very hygroscopic, and relatively volatile (melts at 33 C and boils at 58 C), which is the reason it is
normally found in the form of the hydrochloride salt.
If you make separate solutions in ethanol of hydroxylamine hydrochloride and potassium hydroxide, and then mix, you precipitate KCl, which you can
filter off, and end up with a fresh solution on NH2OH in ethanol which can be used straight away and is much more stable.
I also had an idea to modify the ketazine process to use make hydroxylamine instead of hydrazine. Not sure if it would work...
Preparation of Hydroxylamine using the oxime process ?
Sodium hypoclorite (bleach) and ammonium hydroxide would react to produce chloramine gas (NH2Cl). This unstable poisonous would then be bubbled into a
solution of methylethylketone and sodium ethoxide* dissolved in pure alcohol. The chloramine gas reacts with the methylethylketone and sodium ethoxide
to form O-ethyl-methylethyloxime, with a structure CH3CH2ON=C(CH3)CH2CH3.
The byproduct, NaCl, precipitates out at the bottom at this time, since it is not soluble in alcohol. The progress of the reaction can be estimated by
the formation of the solid byproduct.
Cool the contents and allow an hour for all the NaCl to settle at the bottom, then decant out the liquid into a separate container, leaving the all
solid at the bottom in the first container. The O-ethyl-methylethyloxime then hydrolyzes with a 20% solution of sulfuric acid to form hydroxylamine
sulfate. This would mostly precipitate out at the bottom if only a small amount of the acid solution is added.
*(The sodium ethoxide could be prepared by adding solid NaOH pure alcohol. After several hours, the bottom will consist of solid hydrated NaOH, a
small layer of water saturated with NaOH will form immediately above, and the top layer will consist of pure alcohol with the associated sodium
ethoxide dissolved in it. The top layer would be decanted out. Alternatively, Na2CO3, used to regulate pH for use in pools, and CaO, used as cement
lime can be used instead of solid NaOH, 90% concentrated alcohol can then be used )
http://www.sciencemadness.org/talk/viewthread.php?tid=2656&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=17074
Hydroxylamine (and its compounds) may also be of interest for propellents and explosives:
hydroxylamine perchlorate, hygroscopic solid
melting point between 87.5 and 89degC.
Decomposes at 120degC.
Drop height value of only 2cm, meaning very sensitive to impact.
addition of small ammounts of hydroxylamine perchlorate to several propellents could roughly double their burning rates
US 3748199 (1965)
[Edited on 17-2-2012 by AndersHoveland]
|
|
|
| Pages:
1
2
3 |