napoleon9
Harmless

Posts: 25
Registered: 28-1-2011
Location: viet nam
Member Is Offline
Mood: No Mood
|
|
it differences between diglyme and THF in LAH reaction
when we made a reductive reaction by LiAlH4. We usually used THF solvent, but sometimes we used diglyme solvent. why ? when we used diglyme ? it
differences between diglyme and THF in LAH reaction.
If you used triethylorthoformate protective group, how will you solution protective group ?
thanks
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Diglyme is sometimes more favorable if a higher boiling point is required in reactions requiring more heat. THF is normally easier to use as
extracting the product is easier due to ease of boiling off the THF.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Hmm... It is difficult to say, it is not clear what kind of functional group you want to reduce  As for protecting group, I think orthoester should be stable to LAH, but I didn't check the literature. I agree with
Picric Acid on the boiling point, if your substrate requires forcing conditions then use diglyme but for LAH reductions in general I use THF as
solvent, it has always worked. As for protecting group, I think orthoester should be stable to LAH, but I didn't check the literature. I agree with
Picric Acid on the boiling point, if your substrate requires forcing conditions then use diglyme but for LAH reductions in general I use THF as
solvent, it has always worked.
EDIT: Generally speaking, you can take a look in Greene's Protective Groups in Organic Synthesis to see the compatibility of various protecting groups
with various reagents. You can also check: http://en.wikipedia.org/wiki/Protecting_group
[Edited on 26-4-2011 by Sandmeyer]
|
|
|
napoleon9
Harmless

Posts: 25
Registered: 28-1-2011
Location: viet nam
Member Is Offline
Mood: No Mood
|
|
If product had OTHP group, I washed it by NaOH. But it will give Al(OH)3. So, product will be lost. Please give me some advices.
You will filtered or extracted it.
thanks everybody
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Well, you will have to provide some more information, for examle draw a reaction scheme of what you are trying to accomplish  and attach it as a picture, it is very difficult to discuss otherwise. and attach it as a picture, it is very difficult to discuss otherwise.
I'm puzzled why your THP ether is unstable to NaOH wash (if that's what you mean), it should be stable that's for sure, for more details see: http://www.organic-chemistry.org/protectivegroups/hydroxyl/t...
EDIT: I think I got it.  If you mean that after the reduction with LAH and
consequent bad workup you're faced with a gel which you can't filter - "been there done that" If you mean that after the reduction with LAH and
consequent bad workup you're faced with a gel which you can't filter - "been there done that"  - it is a problem so you have to be careful when quenching LAH, follow the well-established formula: for mixtures
containing n grams of LAH, after the reaction, add n mL of H2O followed by n mL of 15% NaOH and finally then 3n mL of H2O. This will give you dry
white granular precipitate which you can filter. There is also a method of after the reaction adding Na2SO4*10 H2O (solid) until "salts become white",
I never tried this myself. As you have a THP ether, the acidic workup (with 10% H2SO4) will not be an option. I hope this helpz. - it is a problem so you have to be careful when quenching LAH, follow the well-established formula: for mixtures
containing n grams of LAH, after the reaction, add n mL of H2O followed by n mL of 15% NaOH and finally then 3n mL of H2O. This will give you dry
white granular precipitate which you can filter. There is also a method of after the reaction adding Na2SO4*10 H2O (solid) until "salts become white",
I never tried this myself. As you have a THP ether, the acidic workup (with 10% H2SO4) will not be an option. I hope this helpz.
[Edited on 30-4-2011 by Sandmeyer]
|
|
|
napoleon9
Harmless

Posts: 25
Registered: 28-1-2011
Location: viet nam
Member Is Offline
Mood: No Mood
|
|
ok thanks
some magazine give me some advice was it washed by NaOH 0,1M with n mol NaOH = n LAH used.
how do you think about this procedure ?
when do you know finish reaction ? Do you see on TCL ? do you think different value Rf of alkyne and Rf of alkene ?
thank you
[Edited on 1-5-2011 by napoleon9]
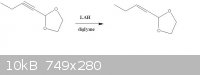
[Edited on 1-5-2011 by napoleon9]
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
There is a difference between wash and quench. What paper are you talking about? "Some magasine" means absolutely nothing. 
Take a look in:
Insect Pheromones. Stereoselective Reduction of β- or ω-Alkynols to the Corresponding (E)-Alkenols by Lithium
Tetrahydroaluminate
Authors: ROSSI, Renzo, CARPITA, Adriano
In: Synthesis 1977; 1977: 561-562
Read the method in the above paper, they use LAH and diglyme, just like you. Unfortunately I can't get the paper so I can't help you more. Take notice
that, in general, LAH gives you E and catalytic hydrogenation Z alkene.
When you talk synthetic routes, papers, reactions and conditions provide as much information as you have otherwise it is very difficult to solve
problems. 
|
|
|
napoleon9
Harmless

Posts: 25
Registered: 28-1-2011
Location: viet nam
Member Is Offline
Mood: No Mood
|
|
I were reduction of 2-(hex-1-yn-1-yl)-1,3-dioxolane by LiAlH4 in diglyme solution. After the reaction was finished, I had 2 ways to wash product:
1/ It washed by HCl 10% solution. After it was extracted with diethyl ether.
2/It was for x g of LAH x g of water, then 3x g of 15% KOH, then x g of water. But I didn't gain white solid. .....
Please give me some advices for this way!
It was moving protected group by HCl 15% for 1h, after it was extraxted by diethyl ether.
thanks
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Post your entire experimental procedure, with all the juicy details in comprehensible English. You can translate Vietnamese to English online: http://translate.google.com/#vi|en|
Keep in mind that dilute acid will hydrolyse your protecting group.
The quench-formula you used is not the formula I suggested - no surprise you didn't get the white solid. If you have difficulty following this formula
then try Na2SO4*10 H2O method I suggested earlier or get another job 
|
|
|
napoleon9
Harmless

Posts: 25
Registered: 28-1-2011
Location: viet nam
Member Is Offline
Mood: No Mood
|
|
I were looking for a procedure :"The reaction was quenched by
addition of aqueous sodium hydroxide 0.1 mol/L (2 mL) at
0 °C followed by addition of ether (10 mL). The aqueous
layer was extracted twice with ether and the organic phase
was dried over anhydrous sodium sulphate. The product
was concentrated in vacuo and utilized in the next step
without further purification."
After this was hydrolysis protecting group procedure:
look below the picture 

Some people were intruduce me that I could be hydrolysis protecting group procedure by CH3COOH 50%, reflux for 2 h.
can you give me some advice, please....
[Edited on 16-5-2011 by napoleon9]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
For acid sensitive groups I usually work up LAH reductions with NH4Cl. Adding sulfate salts (MgSO4, Na2SO4) can also aid with precipitation of the
gel.
But it seems that you are going to remove the protecting group in the next step anyway, so why don't you just use acidic workup?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
napoleon9
Harmless

Posts: 25
Registered: 28-1-2011
Location: viet nam
Member Is Offline
Mood: No Mood
|
|
I will remove the protecting group in the next step. If you are me, how do you do ?
"I usually work up LAH reductions with NH4Cl. Adding sulfate salts (MgSO4, Na2SO4) can also aid with precipitation of the gel"
Can you give me this procedure ? Because I used workup LAH reductions with NH4Cl but It is gel. If you will filter it and wash it with ether, Could
you do lost product ?
Hic, I am worry. Please give me some advice ? thanks everyone 
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I will remove the protecting group in the next step. If you are me, how do you do ? |
Well then just do acidic workup with HCl, you'll get no gel and you're protecting group will be gone too, skipping the next step.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|