Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Ozonelabs- Synthesis of Raspberry Ketone
Our sincere thanks to Klute for the idea to conduct this synthesis and also for providing a good reduction procedure.
Comments are welcome as always.
Regards,
The Ozonelabs Team
Attachment: Rheosmin for pub.pdf (318kB)
This file has been downloaded 6058 times
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Great! I am really pleased someone else tried this out! It's indeed a very fullfilling synthesis!
Not much I can say, recrystallization from water of the unsaturated ketone seems to work very well, perhaps better than the solvent system I used
(EtOH/H2O IIRC). I could suggest using AcOEt/pet ether or DCM/pet teher to recrystallize the saturated ketone, it worked very well for me..
Are you planning on trying other reduction catalysts? NiB on CaCO3 for example? Or preparing analogues? (from vanilline, salicyladehyde, and their
methyl or ethyl ethers, etc)
A few on my to-do's list are the 3,4-methylenedioxyphenyl, 4-fluorophenyl, 4-dimethylaminophenyl, etc
ANyway, great work as always, it was a pleasure to read.. I was so delighted when I saw a new thread on raspberry ketone! 
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Thanks for the commemts klute.
We are planning to make some zingerone very soon and some other variants too.
Initially we tried the Sodium Dithionite reduction however this proved very unsuccessful for us as well as clouding any product we did make with an
unpleasant sulphurous odour.
We did also try your recrystallisation method which did work, however the large yellow needles were absolutely lovely from water so we were very
pleased.
Much obliged klute,
The Ozonelabs Team
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very nice!
Did the unsaturated ketone have a smell?
Sorry if this is an obvious question, but how is reduction of the ketone prevented, or why is reduction of the alkene so selective? I tried looking it
up but couldn't find anything right away. Are some of the side products the alcohol version?
Moved to Prepublication as it seems like a very decent piece of work!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
chemoleo
|
Thread Moved
3-9-2009 at 14:18 |
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Actually, yes the unsaturated ketone did smell, personally I'd describe it as a 'bubblegum'/raspberry scent- not unpleasent by any means!
Catalytic hydrogenation of Carbonyl Groups is a slow reaction, though it does occur- the reduction of the alkene to the alkane is far more rapid.
Information on the alcohol product (4-(3-hydroxybutyl)phenol) is sparse, though it is a reasonable possibilty as an impurity. We should hopefully be
performing GS/MS, IR and HNMR on the product- so will report back with any further findings, thankyou for raising the point.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The file does not open here (Acrobat 5).
Tim
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
Im interested in the reduction of that double bond with hydrogen.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
See the raspberry ketone thread in Org Chem section for various catalysts capable of reducing the double-bond without reducing the carbonyl.. NiB
supported on various supports, Cu/SiO2, CoB, Rh/C, Pd/C, etc
http://www.sciencemadness.org/talk/viewthread.php?tid=9706
[Edited on 6-9-2009 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Our apologies- it seems as though a part of our PDF wasn't published- Under the References heading-
Our sincere thanks to the School of Chemistry at the University of Southampton for help with analysis of the materials, and also for
personal correspondence without which this project would not have become a reality.
Regards,
The Ozonelabs Team
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
This is a beautiful synthesis with acetone, but I wanted to try with vanillin a variety of other ketones to create a variety of zingerone analogues.
But I am running into difficulty with my first variation.
2,6-dimethylheptan-4-one (diisobutyl ketone) is not water-miscible as is acetone, so the initial aldol condensation is slow. Stirring the mixture for
two days only produced about 5% of a second product by TLC, and refluxing (at 163 degrees) produced a lot of tarry gunk.
I wonder if anyone has any recommendations.
Options might be to repeat with an excess of some solvent... methanol or ethanol, or maybe to try an acid-catalysed aldol. But I am unfamiliar with
this route.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Use NaOH in MeOH instead of NaOH in H2O, and only 3-fold excess of the ketone (instead of the ~6-fold in acetone example).
The reaction will have to be monophasic or else all the vanillin sticks in the aq. phase in the form of the phenoxide, and because diisobutyl ketone
is not particularly soluble in water the reaction might never go anywhere. If you still have the reaction mixture you might try adding a quat PTC
catalyst and stir vigorously at 40-60°C.
The product would be a compound not yet described in the literature, so I can not help you with a literature synthesis. Due to the alkyl branching, it
is possible that the product will be either a viscous oil at room temperature or a hard to crystallize solid, in which case you will have to find an
alternative work up instead of the recrystallization described above. Flash chromatography anyone?
"about 5% of a second product by TLC"
You must have supernatural powers to estimate conversion from a TLC?
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Right, so redo in a big excess of MeOH.
Do you have a review of acid-catalysed aldol, or can you offer any comments?
Well, the new spot (Rf 0.77) was only just visible as a UV-absorbing spot wheras vanillin (Rf 0.71) was huge. Solvent CHCl3/MeOH/H20 74/15/1. Calling
it 5% is a guestimate.
Edit:-
5g vanillin, 4g NaOH, 50 ml DIBK and 500 ml MeOH formed a clear, very light yellow homogeneous mix. I'll check it daily and post any results.
[Edited on 15-1-2010 by Paddywhacker]
|
|
|
medchem
Harmless

Posts: 41
Registered: 12-12-2009
Member Is Offline
Mood: No Mood
|
|
though reaction involves simple methods of aldol followed by reduction, it's interesting. And these compounds had a range of biological activities
like antiinflammatory, anticancer etc.
And regarding synthesis of unsaturated ketone, use of base KOH/NaOH (1.2 eq of ketone) in solvent system of MeOH/water (5:1) will work fine. And
reduction of the alkene is possible by H2, pd/C.
Crystals of unsaturated chalcones are nice in appearance 
BTW, are you synthesizing gingerone for any biological purposes or only synthesis? Just curious!
Good luck!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The only review I know of is the one in Organic reactions, 16. The whole volume 16, that is over 400 pages, is dedicated to the aldol
reactions of all types, base or acid catalysed. This volume is unfortunately unavailable in electronic form, so you will have to visit a library to
get it. It is from year 1968 though, but the best you can get. There are a bunch of new reviews about the asymmetric aldol reactions, but these are
not really relevant to the topic. Neither are the directed aldol reactions for which there are also numerous reviews.
| Quote: | Edit:-
5g vanillin, 4g NaOH, 50 ml DIBK and 500 ml MeOH formed a clear, very light yellow homogeneous mix. I'll check it daily and post any results.
|
I would stick to the original ratios if I were you. There is a reason for using 1.1-1.2 eq. of NaOH when using hydroxybenzaldehydes (1 eq. is lost in
the deprotonation of the phenolic group + <0.2 eq. are needed to catalyse the aldol). Instead you used 3 eq. of NaOH which might cause troubles due
to self condensation of DIBK and make the work up more of trouble that it should be (though at least this ketone is less reactive than acetone). Also
using a 10-fold excess of the ketone is overkill - just an additional problem that will make the work up more troublesome.
Another advice is to do trial reactions at <10 mmol scale. There is no need to waste precious reagents just to try out if something works or not.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
medchem
Harmless

Posts: 41
Registered: 12-12-2009
Member Is Offline
Mood: No Mood
|
|
[quote=169725&tid=12780&author=Nicodem] Quote: Originally posted by Paddywhacker  | e
I would stick to the original ratios if I were you. There is a reason for using 1.1-1.2 eq. of NaOH when using hydroxybenzaldehydes (1 eq. is lost in
the deprotonation of the phenolic group + <0.2 eq. are needed to catalyse the aldol). Instead you used 3 eq. of NaOH which might cause troubles due
to self condensation of DIBK and make the work up more of trouble that it should be (though at least this ketone is less reactive than acetone). Also
using a 10-fold excess of the ketone is overkill - just an additional problem that will make the work up more troublesome.
Another advice is to do trial reactions at <10 mmol scale. There is no need to waste precious reagents just to try out if something works or not.
|
I totally agree with this comment. 
It's good to have small scale trial.
but in case of excess base, you can neutralize with 2N HCl after completion of reaction. It will be better option while doing workup. (If you are
lucky you can get precipitate after neutralization with HCl, which will be easier to purify)
[Edited on 17-1-2010 by medchem]
[Edited on 17-1-2010 by medchem]
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Progress report. The yellow colour intensified over a day at ambient temperature, but seems not to have gotten any darker after another day. At 2
days there is still only vanillin by TLC (Rf 0.54 with hexane/EtOAC 50:50) by UV. Nothing by iodine stain. Should I wait longer or reflux, or try
again?
I was interested to note that there was no sign of Cannizzaro with the aromatic aldehyde.
Thanks for the advice. This counts a small-scale to an oldschool chemist like myself, but I really should get with the microscale program.
Medchem, this zingerone analogue looked accessible, and was not listed according to chemspider, and SAR is an endlessly fascinating crapshoot, so this
it was.
Methyl isobutyl ketone is less hindered then the DIBK that I used. Maybe I'll try next with that, but I don't like to be an early quitter.
Final report, 02 Feb 2010:- I figured that I was led astray by the compound co-eluting with vanillin on the TLC and worked it up.
The intermediate had a melting point of 74-76 Celcius.
In-situ reduction with zinc powder and acetic acid in ethanol with Pd/C catalyst gave an oil that recrystallised from ethanol in the freezer but
remelted at room temperature. A dilute aqueous mouthwash did evoke a hint of ginger.
Without instrumental characterization, of course, I might have nothing except contaminated vanillin. I will try and get an IR spectrum once
university starts.
[Edited on 1-2-2010 by Paddywhacker]
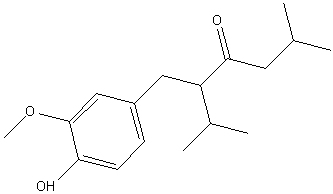
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
In highschool, my first chemistry class failure smelled like a perfect banana ester. Thus, I'd love to do similar myself - thanks for the
re-inspiration!
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
TonedTony
Harmless

Posts: 1
Registered: 29-4-2011
Member Is Offline
Mood: No Mood
|
|
All of the information above is interesting, thanks to all the contributors. I'm looking to find out any types of tests run about raspberry ketone
altering lipid metabolism or increasing norepinephrine induced lipolysis. Can anyone point to studies?
Also, is there a better place to buy this? I've found it here: http://www.nutraplanet.com/product/nutraplanet/raspberry-ket... but didn't know if there was a better source. Thanks in advance for all of your
help!
|
|
|