| Pages:
1
2 |
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
p-hydroxybenzaldehyde?
Hi 
I'm wanting to one day make some raspberry ketone but first I'll need some p-hydroxybenzaldehyde, it is far too expensive for me to buy from a chem
supplier (plus the odd looks I'd get) so I was wondering if it would be possible to start from benzaldehyde and then a Baeyer-Villiger oxidation to
phenyl formate, then a Fries rearrangement to p-OH-benzaldehyde.
Before I look too far into this, would the Baeyer-Villiger even work on benzaldehyde? I've read that aldehydes can form formates but also that it may
just oxidize it to benzoic acid.
|
|
|
cheeseandbaloney
Harmless

Posts: 21
Registered: 4-4-2008
Member Is Offline
Mood: No Mood
|
|
I would think that since -H has a higher 'migratory aptitude' (faster than a -Ph group) Baeyer-Villiger oxidation of benzaldehyde would primarily
yield benzoic acid...
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Anise oil is used in several references as a precursor to p-methoxybenzaldehyde, use google and you'll find them
quam temere in nosmet legem sancimus iniquam
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I was after the hydroxy benzaldehyde but demethylating the methoxy shouldn't be too bad, thanks 
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
You could start by sulfonating toluene to p-tosic acid, followed by fusion with caustic soda to form p-cresol, followed by oxidation to
p-hydroxybenzaldehyde.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Wouldn't p-cresol be pretty sensitive to oxidation?
|
|
|
Intergalactic_Captain
Hazard to Others
  
Posts: 228
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
Took ~7 months of lurking, but the password reset finally worked and I have my account back... Hopefully my first post since my last is helpful here;
You could start from Chavicol , isomerization via KOH followed by cleavage with cold, dilute, basic KMnO4 (a textbook sort of route) should work (ozonolysis would be
better though).... Need to look around to see how viable chavicol would be though, most searches seem to bring up methyl-chavicol (estragole) en
masse.
I'm thinking, though, once again, of acetaminophen... Hydrolysis via HCl (~5 hours, 20% HCl, around 5mL/g worked for me a while ago) will provide you
with p-aminophenol hydrochloride...This is then subjected to a "sandmeyer reaction with CuCN to yeild the benzonitrile, which will be subjected to a
stephen reduction to yeild your p-hydroxybenzaldehyde.
...Now, the above route may be condensable to a one-pot for the hydrolysis and sandmeyer (worked for me with KI and apap, but then iodide is plenty
reactive in this reaction - not sure if -CN is the same or if that was a special case). I also am unable to provide refs on the stephen reduction, as
my journal access is out for the near future - However, I'm sure some other members can tell you whether or not the pathway is feasable. Not
elegant, but relatively cheap by the gram if done on a mole or higher scale.
If you see me running, try to keep up.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Phenol can condense with glyoxylic acid to form p-hydroxy mandelic acid. This can be oxidized and decarboxylated easily to get the benzaldehyde.
There is a paper on this somewhere in the references thread and several patents.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Found this at the university library. The second procedure looks particularly well suited to the home chemist. Source: Kirk-Othmer Encyclopedia of
Chemical Technology, Third Edition, Volume 13, pg 74.

[Edited on 18-8-2010 by crazyboy]
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I don't have access to phenol in Australia unfortunately so the Riemer-Tiemann route is out, as well as the condensation with glyoxylic acid and
chavicol would also be pretty hard to get.
I bought a lot of paracetamol when it was on sale so I have plenty of that to play with, I could get some ferricyanide and make CuCN as well so that
seems pretty good.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
para-Hydroxybenzaldehyde is relatively cheap and uncontrolled substance. If you just need it for further use, you are better of by just buying it. I
doubt any chemical seller will give you troubles if you just order it. But I think it is a good idea if you actually try to make it by your own first.
The Reimer-Tiemmann formylation of phenol gives the ortho-hydroxybenzaldehyde as the main product so it has not much use here. Though there are
variations, tested on phenol as well, that use trialkylamines, PEG, modified cyclodextrins, or other additives, which supposedly make it
para-selective (UTFSE for some of the references). Regardless, the Reimer-Tiemmann formylation is a messy and often poor yielding reaction while the
separation of regioisomers is not something appropriate for beginners with little experience with fractionation or other separation techniques.
What you could do is try the modified Duff formylation of phenol with hexamine in acetic acid. Phenol can be prepared from aspirin (UTFSE for more
info).
For the sake of experimentalism you could first try to formylate aspirin or salicylic acid by using the acetic Duff formylation. This should give
3-carboxy-4-hydroxybenzaldehyde as the major product and 3-carboxy-2-hydroxybenzaldehyde as the minor. They should be separable by recrystallization.
Already refluxing a mixture of salicylic acid and hexamine in water gives a 22% yield of the two aldehydes in a 2.3 : 1 ratio (see J. Chem. Soc.
(1932) 1987 by Duff himself). I'm not aware of any attempts of this formylation done in acetic acid, but the yields should improve greatly. Given
these products are solids (unlike p-hydroxybenzaldehyde) they are easier to isolate, purify and characterize in an amateur setting and the experience
gained would be of great value for further work.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I'd still get an odd look from the chem supplier, they always assume I'm making either drugs of explosives. Plus making it from scratch is more fun

Aspirin is awfully expensive, I do have about 48g of it I bought to make methyl salicylate, what are the yields like for it? I didn't think they were
all that good so I didn't want to use it.
I was thinking of trying the oxidation of benzoic acid with CuO for some phenol though but I'm not sure how easy it would be.
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
As suggested by manimal earlier in this thread, I am currently attempting to following his route to 4-hydroxybenzaldehyde.
| Quote: | Originally posted by manimal
You could start by sulfonating toluene to p-tosic acid, followed by fusion with caustic soda to form p-cresol, followed by oxidation to
p-hydroxybenzaldehyde. |
So my question is, has anyone any experiance/references/suggestions on how to oxidize the p-cresol to the aldehyde?
Have searched the net but have yet to find anything specific; oxidizer, method etc.
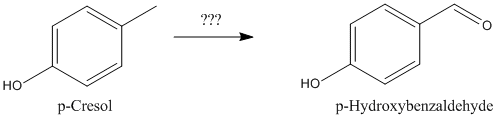
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
P-Propenyl Anisole is easily acquired in many locales. Anise is loaded with it. Once introduced, it grows wild. Just steam distill it out.
Phenylisopropylamines derived from this material are disappointing and toxic. But, it might make a good starting material for your
P-Hydroxybenzaldehyde.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Hmmm perhaps p-crescol could be acetylated first then it would be a bit more resistant to oxidation, maybe acidic dichromate could work if not then
some of the methods discussed in the benzaldehyde thread could.
Yeah anisole could be a good option, my oxidation of eugenol to vanillin failed though so I'd have to work out a good method first (without
nitrobenzene if possible, it just smells too good for how toxic it is  ) )
I'm going to have a shot at making phenol from benzoic acid first, I've read up on the reaction a bit and it seems like I could just have a 3neck RBF
with thermometer, air inlet(fish tank pump) and condenser, the air could be bubbled through the melt and the phenol distilled straight off with hot
water(or air) in the condenser to keep it a liquid.
I could then hopefully react it with acetic formic anhydride to give phenyl formate, then a fries rearrangement to the aldehyde.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
In the crazyboy's book scan there is written that salicylic acid can be reduced to salicylaldehyde by electroreduction. That means we can
electroreduce 4-hydroxybenzoic acid obtained from heating potassium salicylate with potassium carbonate. Maybe sodium salts would work?
Preparation of 4-hydroxybenzoic acid:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2...
If the electroreduction would be easy that could be one of easier ways of obtaining it.
|
|
|
dean stark
Harmless

Posts: 12
Registered: 17-3-2011
Member Is Offline
Mood: No Mood
|
|
From p-nitrotoluene...
Reduction/oxidation with sodium disulfide or polysulfide to p-aminobenzaldehyde [1,2,3]
Diazotization/decomposition to p-hydroxybenzaldehyde [4,5]
[3] discusses preparation of polysulfides starting from sodium monosulfide.
[2] and [4] are attached. I don't have access to [1].
__________________________________________
[1] "Preparation of p-Aminobenzaldehyde, and the Mechanism of the Reactions of Sodium Polysulphides with p-Nitrotoluene" by Beard and Hodgson
[2] "Mechanism for Reaction of p-Nitrotoluene with Sodium Polysulfide to Form p-Aminobenzaldehyde" by Ogata, Kawasaki, Sawaki, and Nakagawa
[3] US2795614
[4] "A Simple Preparation of Phenols from Diazonium Ions via the Generation and Oxidation of Aryl Radicals by Copper Salts" by Cohen, Dietz
Jr., and Miser
[5] GB232392
Attachment: Archive.zip (1.3MB)
This file has been downloaded 710 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The para orientation is fairly specific to potassium salts, sodium gives mainly the ortho isomer except at higher temperatures.
While I like organic electrochemistry, it is often fussy about the conditions used. In particular electrode substitutions are likely to cause
problems, the electrode overvoltage is quite important and the electrode surface condition can strongly influence yields. On the other had electricity
is fairly cheap, if the starting material is also low cost even a low yield method may be satisfactory. Note that some reductions take benzoic acids
to the alcohol, which could then be oxidised to the aldehyde.
Quote: Originally posted by Random  | In the crazyboy's book scan there is written that salicylic acid can be reduced to salicylaldehyde by electroreduction. That means we can
electroreduce 4-hydroxybenzoic acid obtained from heating potassium salicylate with potassium carbonate. Maybe sodium salts would work?
Preparation of 4-hydroxybenzoic acid:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2...
If the electroreduction would be easy that could be one of easier ways of obtaining it. |
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
p-hydroxybenzoic acid can be obtained by hydrolysis of their esters, available OTC as parabens, such as methyl paraben.
|
|
|
no_dream
Harmless

Posts: 14
Registered: 15-8-2011
Member Is Offline
Mood: No Mood
|
|
Experimental: US patent 4,429,163
The ratios were p-cresol 12g, CoCl2 knife tip, KOH 18g, methanol 40ml. Apparatus was flushed with oxygen, a inflated baloon with O2 was attached and
the water bath temperature was held about 65 degrees. Stirring and heating was continued for 6 hours.
50ml water was added and the mixture filtered hot.
Then MeOH was distilled off at normal pressure. The remaining solution solidified on cooling. I was suction filtered and washed with cold water.
Almost all crystals dissolved. The appearance was not like crystals, more like an ice/water slurry. After addition of HCl an dark oil appeared and
smelled much of cresol. No crystals. Everything was discarded.
Second experiment US pat 4,748,278 scaled down 1/20.
p-cresol 21,6g, CoCl2 0,5g, NaOH 24g, 65ml MeOH. The mixture was stirred and heated for a total of 12 hours, and the absorption of pure oxygen was
about 3 litres of gas(4,5l theoretic absorption). Gas pressure was about an meter of water column. 60 mls water added and filtered hot. Filter washed
with some MeOH, remains black fine precipitate.
Methanol and some water was stripped under vacuum, the maximal bath temperature was 80 degrees and 50 deg. for vapors. Everything solidified on
cooling. Some water was added and the flask heated to dissolve the solids. The resulting liquid was orange-red in color and put in a beaker where it
solidified on cooling, more it was cooled in an ice bath. After vacuum filtration the result was an orange slurry and orange filtrate. The cresol
smell was practically absent. On washing with ice cold water with a little NaOH the product almost dissolved...
EDIT: What almost dissolved was the Na salt of the hydroxybenzaldehyde, which must be then neutralized with acid.
Seems something is wrong, maybe the cresol. It is old and discolored, the appearance are dark red crystals and liquid. Vacuum distillation would
probably solve it but the smell...
[Edited on 5-8-2012 by no_dream]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by no_dream  | | Methanol and some water was stripped under vacuum, the maximal bath temperature was 80 degrees and 50 deg. for vapors. Everything solidified on
cooling. Some water was added and the flask heated to dissolve the solids. The resulting liquid was orange-red in color and put in a beaker where it
solidified on cooling, more it was cooled in an ice bath. After vacuum filtration the result was an orange slurry and orange filtrate. The cresol
smell was practically absent. On washing with ice cold water with a little NaOH the product almost dissolved... |
I don't really understand this work up. Where is the neutralization to liberate the phenolic compounds?
How did you monitor the reaction? Did you do at least some TLC monitoring?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
no_dream
Harmless

Posts: 14
Registered: 15-8-2011
Member Is Offline
Mood: No Mood
|
|
The reaction was monitored only by O2 consumption.
The filtration should remove phenolic compounds from the sodium salt of hydroxybenzaldehyde, according to the patent. The salt is then dried and
neutralized with acid. But because almost all dissolved, this step was yet not performed. The salt should be sparingly soluble in cold water and
almost colorless. It was orange and pretty soluble. The filtrate with almost all dissolved product is cooling and waiting if something crystallizes
out.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
The p-hydroxybenzaldehyde which I have is a light reddish-pink, and even after recrystallizations from boiling water it still has an off color tint.
Perhaps instead of that final wash in which most of your product dissolved away you may consider neutralizing the crude phenolate, and purifying the
product by other means ( recrystallization from water or water and ethanol).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The alkali salts of p-hydroxybenzaldehyde are likely soluble in water, so you should not be washing them with water. In my experience the alkali
phenolates can be very colored if they have conjugated fromyl, acyl or nitro groups. The colors can range from yellowish to intense canary yellow to
orange or even red.
You should be able to remove and recycle the unreacted p-cresol by diluting with water and neutralizing the reaction mixture, then doing a steam
distillation. This should give you a recovery of a fairly pure p-cresol (p-hydroxybenzaldehyde does not come over with steam). You can skip the steam
distillation if you don't care about the p-cresol recovery, but the recovery also gives an idea of the conversion, given that you don't appear to have
any means to follow the reaction.
The p-hydroxybenzaldehyde can then be extracted with ethyl acetate (as in the patent examples), the extract washed with 1% NaHCO3 to remove the
potentially formed p-hydroxybenzoic acid. Another wash with water and then rotavap to get the crude product which could be purified by vacuum
fractionation, or via a bisulfite adduct, or - if already sufficiently pure - by recrystallization.
Good luck and keep us updated on this interesting project.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
no_dream
Harmless

Posts: 14
Registered: 15-8-2011
Member Is Offline
Mood: No Mood
|
|
There is no need for recovery of cresol. I have about a kilogram of old oxidized cresol and would to convert it without purification.
The procedure should be simple as possible without using much solvents for extraction and similar.
This tests proved the oxidation works, because the uptake of oxygen. The uptake slowed down with time, and was nearly zero about ten hours. There were
no leaks in the apparatus.
The solvent extraction is one thing i like to avoid, and the patent US4,748,278 shows the way of filtering the Na salt off. First it seemed the K salt
is too soluble so the Na salt was prepared.
Patent citation:
...The aqueous solution from the bottom of the column is cooled with stirring, preferably to approx. 5°. The sodium salt of p-hydroxybenzaldehyde, of
the formula ... , crystallizes out in the form of leaflets, whereas the potassium salt crystallizes with one molecule of water of hydration. Both can
be filtered off easily.
The working up of the mother liquor described in the example shows that 98% of the p-hydroxybenzaldehyde formed has been precipitated as the sodium
salt in the procedure described.
....
The aqueous solution remaining after the methanol had been distilled off was cooled to 5°C, whereupon the sodium salt of
p-hydroxybenzaldehyde crystallized out in the form of leaflets. The crystals were thoroughly suction drained and washed with a little sodium hydroxide
solution. The nearly colorless sodium salt of p-hydroxybenzaldehyde was dried
End of citation.
Continuation of the experiment.
About a teaspoon of orange crystal slurry remained on the filter. This is still wet and seems not to dry out in air even after 24 hours. The filtrate
in the fridge shows no signs of crystallization.
Would concentrating the filtrate to obtain the crystals back and then wash them with 50 per cent NaOH help? The patent doesn't state the wash
concentration. Well, maybe the amount of wash water (about 150mls of iced water with a dash of 50pct NaOH) was excessive, but the thought was to get
rid off the orange color.
|
|
|
| Pages:
1
2 |