| Pages:
1
2
3
4 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There have been reports of runaway reactions which have been quenched to avoid further " complications " . Think about it and you will
realize that the potential is there for a thermally induced
" cook-off " , when the batch size is large and the proportions of materials present is not a safe mixture to be subjecting to a sudden
temperature spike which can sometimes keep right on going to reach the ignition point . Picric acid is probably the safest trinitration of an
aromatic , but I personally wouldn't ever perform its synthesis operating on the assumption that there is no danger about the process . One
safety enhancement is provided by keeping the temperature high enough to guarantee that the nitration reaction proceeds without accumulation of
unreacted nitrate or nitric acid which would aggravate the danger if the reaction suddenly surged and became uncontrollable , going into an oxidation
decomposition mode which is autocatalytic and therefore self-accellerating . That uncontrolled process is limited only by the quantity of material
present , and so it becomes a matter of
whether or not there is " critical mass " for the reaction mixture .....and if that condition is present , then the geometry lesson for the
day pertains to " blast radius " 
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
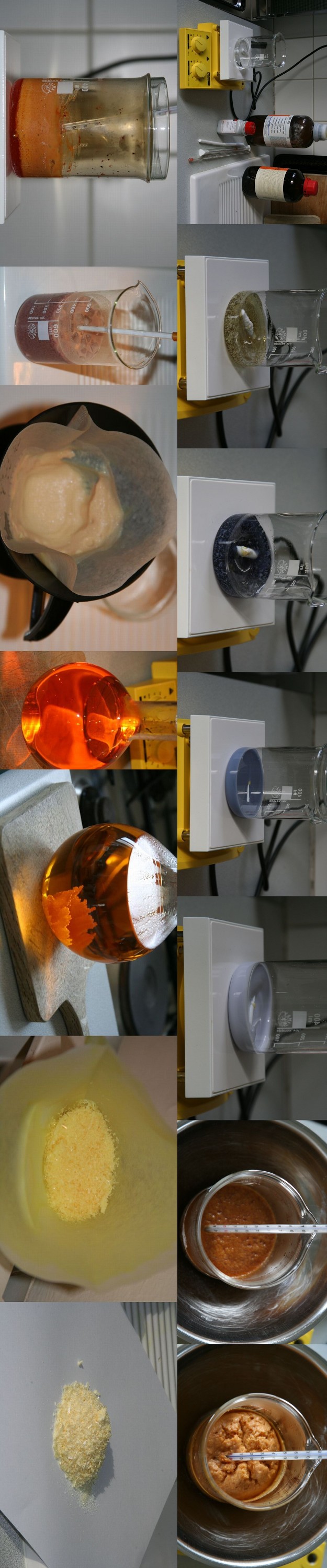
Rosco's TNR procedure
I prepared TNR using Rosco's procedure (page 1 of this thread) and same starting quantity, i.e. 10 gr commercial grade resorcinol. I thorougly
followed the times and temperatures indicated, just taking some liberty from the recipe by accelerating somewhat HNO3 addition (68%, then 100%) after
having observed than the temperature rise would be much weaker after dropwise addition of the first 10 ml of 68% HNO3. But at no time did the
temperature overpassed 22°C.
After slow crystallisation from boiling distilled water, the crop of dry crystals was 16,2 gr iso 20 gr expected. However, I didn't try to recover the
part of TNR still dissolved in the cold solution, although by evaporating a few ml of it, it left crystals in a non neglectable amount. Although TNR
is notably less soluble in water than picric acid, the literature mentions 0,45 gr per 100 ml at 15°C. As I separated the crystals when the solution
had reached 4°C, I assume that about 2 gr remained in the discarded 900 ml liquid... which would makes a total crop around 18 gr, still 2 gr short
compared to Rosco's record.
EDIT: made pic slightly smaller and rotated it to avoid page stretching
[Edited on 8-23-2010 by Polverone]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Your picture collection is very well done. I was looking at various methods of styphnic acid production just now and found a "NITRITE" synth. I have a
collection of material from patents and sources that have proven to be worthwhile keeping (Roscoe's stuff, etc) and this had an old date and no lab
notes....For what it's worth, I don't know it's background (what patent it came from, etc) but since this thread has had so much material added to it
over the months...here it is:
Step 1. Preparation of Dinitroresorcinol monohydrate
To 16 liters of water add and dissolve 440 grams of resorcinol, then 452 grams of 98% sulfuric acid. Immediately thereafter, add 6800 grams of
crushed ice to mixture, and then stir the mixture vigorously until the internal temperature of the mixture reaches about 0 Celsius. Then prepare a
solution by dissolving 600 grams of sodium nitrite into 3200 milliliters of water, and then add this sodium nitrite solution to the
sulfuric/resorcinol mixture over a period of 6 minutes while vigorously stirring the mixture and maintaining its temperature below 5 Celsius. After
the addition of the sodium nitrite solution, continue to stir and cool the reaction mixture below 5 Celsius for 30 minutes. Note: A precipitate will
begin to form. After the 30 minute period, filter-off the precipitated dinitroresorcinol monohydrate, and wash with several hundred milliliters of
cold water (use gravity filtration).
Step 2. Preparation of Styphnic acid
Place 3000 milliliters of 40% nitric acid into a beaker, and then gently heat this mixture to about 30 Celsius. Thereafter, carefully mix the moist
filter cake, prepared at the end of step 1 to the nitric solution over a period of about 30 minutes, while stirring the nitric acid and keeping its
temperature around 30 Celsius. Immediately after the first addition of the filter cake, nitrogen oxide gases will be evolved, followed by the
formation of a foam (the foam will dissipate after about 10 minutes). After the addition, raise the temperature of the mixture to 95 Celsius, and
then hold this temperature for 1 hour. After heating for 1 hour, remove the heat source and allow the mixture to cool to room temperature. Note: A
precipitate will form. When the reaction mixture reaches room temperature, filter-off the precipitated product, wash with 300 milliliters of 2%
nitric acid, and then with 600 milliliters of cold water. Then air-dry the product. The result will be pale yellow crystals, well suitable for use
in preparing lead styphnate, or Styphnic acid compositions.
As you can see it was designed to lab production size. I do not know if scaling it down would intail problematic issues but if it worked on a smaller
scale it would be interesting as it cuts down on materials, etc.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by pdb
I thorougly followed the times and temperatures indicated, |
Really ?
| Quote: |
But at no time did the temperature overpassed 22°C. |
Then your cooling was too agressive for the reaction to
proceed from its own exotherm . The way to patch things
would have been to remove all cooling and even nudge the
reaction with a little supplemental heat . When I use the term " keeping below a certain temperature " that is not to say that any temperature way
below that will be fine , but
it is usually a temperature limit very near where you want to be with the reaction , but observing it as a point where
the reaction may not self-regulate very well or may show signs of decomposition byproducts . Generally for nitrations
you want to stay just under that temperature to keep the reaction rate steady , but not to exceed that temperature
during that time when the reaction is being regulated by rate of addition . When the reaction is not maintaining its
temperature , then you have to adjust the conditions so
that it does stay on track , understanding that there is
an optimum thermal curve the nitration should follow for
best results .
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
I do understand your point and agree. However, in my mind, 22°C was not that far from 25°C... Maybe this would deserve a wording precaution next
time
BTW, isn't it a characterisation of a major difference between O- and C- nitrations ? E.g., NG is prepared while keeping temperature under 25° or
30°C (depending on authors) as a safety precaution, and good yields are still obtainable at quite lower temperature, while, in order to secure high
yields, phenol-like nitrations have to navigate as close as possible of an upper limit, beyond which the likelihood of runaway is too high.
[Edited on 6-2-2006 by pdb]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by pdb
I do understand your point and agree. However, in my mind, 22°C was not that far from 25°C... Maybe this would deserve a wording precaution next
time |
Indeed I could have done a better job of writing up the
details , but just had a paragraph in a lab notebook to
reference . Next time I do the synthesis I will be more thorough with my notes . A few minutes is not very specific
for example , and the minutes do count on a finite curve
where a reaction is affected by temperature and time .
| Quote: |
BTW, isn't it a characterisation of a major difference between O- and C- nitrations ? E.g., NG is prepared while keeping temperature under 25° or
30°C (depending on authors) as a safety precaution, and good yields are still obtainable at quite lower temperature, while, in order to secure high
yields, phenol-like nitrations have to navigate as close as possible of an upper limit, beyond which the likelihood of runaway is too high.
|
Yes good yields can be obtained at lower temperatures
with things that are easily nitrated but also easily oxidized ,
but the reaction rate slows so it becomes something of a tradeoff to choose a good working temperature . But it generally requires driving aromatic
nitrations pretty hard to
effect their complete nitration , on the order of 3 times the temperature you would need for completely nitrating an aliphatic . Even at the elevated
temperature , it is safer
for the nitration of the aromatic . You actually can completely nitrate an aromatic at the lower temperatures , if you don't mind waiting three days
or even a week for the nitration to complete  I never actually tested this but
it would seem so . I never actually tested this but
it would seem so .
BTW the picture file you posted above won't completely download for me , maybe a corrupt file or the forum server
is dropping out . It has been acting wierd for a couple of days , dropping out completely at times . Could you maybe reattach the file and see if
that helps anything .
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Something's wrong on your side, Rosco, as his photo loads just fine on my machine.
[Edited on 7-2-2006 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The pictures are loading fine for me now so the file
is okay . I think it was the server acting out a bit
from time to time and I just tried during a temporary glitch .
It does load the first picture and then jump to a blank screen
until I wave the cursor of my mouse around a bit in the upper right part of the blank field and see that box for
resizing the image . When I click that box then the first
frame reappears and all is well scrolling to the other pictures also .
|
|
|
ppoowweerr
Harmless

Posts: 5
Registered: 13-8-2007
Location: USA
Member Is Offline
Mood: No Mood
|
|
I am working on some physical properties of lead styphnate, and since TNR is somewhat difficult to purchase, I had to make some before I can begin
working with it. I have had some difficulty getting a decent product but thanks to rosco, I have now achieved very nice pale yellow hexogonal
crystals. The only problem is that I am not sure about the best means to prepare it for impact detonation. I think I have too much h2o present. I used
an ATR IR and got an awesome spectra with even the fingerprint region is all accounted for compared to a spectra I found online. So now my questions:
Will simply using a vacuum oven work to dry? What does the dinitro version look like on IR and to the eye? And thanks again to rosco and othersw who
have posted on this thread.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Somebody needs to edit the image widths above
to about 550 pixels , and post the images sequentially ,
so they scroll vertically , instead of the horizontal panorama that appears now
and screws up the text formatting for the whole page .
Good pictures .....but the formatting stinks .
[Edited on 1-9-2007 by Rosco Bodine]
|
|
|
tito-o-mac
Hazard to Others
  
Posts: 117
Registered: 30-6-2007
Member Is Offline
Mood: No Mood
|
|
Was the residue crude or pure?
|
|
|
ppoowweerr
Harmless

Posts: 5
Registered: 13-8-2007
Location: USA
Member Is Offline
Mood: No Mood
|
|
my residue was pure. It was pale yellow hexagonal crystals and i have been drying it in a low temp oven.
The IR was perfect and the MP was 177-181 C. I was very happy with the small range and Merck 12th Ed
says 180 MP. I am going to run it through a bomb calorimeter just for fun, tomorrow I hope. If not tomorrow then next week. Oh and by the way fuming
red nitric is expensive. Thanks
|
|
|
ppoowweerr
Harmless

Posts: 5
Registered: 13-8-2007
Location: USA
Member Is Offline
Mood: No Mood
|
|
I havent done any math yet so I dont have any numbers but I did get to run about .75g of TNR
in the bomb calorimeter. It yielded a net change of 0.8 C degrees in 2 L of h20. when I get the
numbers worked out tomorrow (or this weekend) I will be able to compare to published numbers
with mine for another test of purity.
What is the best method adding lead to make lead styphnate?
|
|
|
ppoowweerr
Harmless

Posts: 5
Registered: 13-8-2007
Location: USA
Member Is Offline
Mood: No Mood
|
|
Thanks for all of your help, guys. I have finally made a satisfactory product of lead styphnate that yielded a surprisingly large detonation. thanks
all
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
reciprocal solubility NH4NO3 - NaNO3 and NaNO3 - KNO3
Quote: Originally posted by quicksilver  | | Quote: | Originally posted by Rosco Bodine
The use of nitrates as concentrated solutions does simplify addition of the nitrate to the sulfuric acid solution of
the sulfonated organic material to be nitrated . I have mentioned that using
a solution of two or more different nitrates
can make possible an even more concentrated solution having less water content which would dilute the nitration mixture . Such solutions of mixed
nitrates have been developed to provide a liquid oxidizer phase having low water content for use in manufacture of emulsion explosives . Some of
these solutions are
essentially a eutectic salts mixture which
also exhibit a enhanced cosolubility in the presence of a small amount of water , similarly as they have a much lowered melting point even in the
absence of water altogether.
I know I have seen other compositions mentioned and I will share any others I find . |
I was unawair of the "mixed-nitrates" concept...and PLEASE; if you do find material related I would deeply apprieciate seeing it!
- I too use the PATR but find that patents are making much more impact in finding answers, new proceedures, & use of my time. Some of the most
interesting stuff I have found have come from US patents thus far.
As I have the same trouble accessing UK patents, as you have noted yourself, that site is a real pain. But the older, valuable patents (where they
were nitrating everything under the sun) and the older techniques, seem to originate in the UK. |
Here are some reciprocal solubility charts for a couple of the binary systems of mixed nitrates which indicate the co-solubility enhancement which
occurs at certain temperatures anyway for certain unique proportions of salts in mixture in water solution. There are definitely other binary and
tertiary and possibly quaternary systems where a peculiar increase in water solubility occurs for certain specific proportions of particular nitrates
in mixture. Calcium nitrate and ammonium nitrate exhibit reciprocal solubility, and I think it is also true for magnesium nitrate with other
nitrates which would be very interesting. Where the indexing of such reciprocal solubility data is to be found I do not know.
To anyone who may be helpful with reciprocal solubility data references for nitrates, please add to these references.
Attachment: Reciprocal Solubility of NH4NO3 and NaNO3.pdf (119kB)
This file has been downloaded 906 times
Attachment: Sodium Nitrate Solubility and Reciprocal KNO3 Solubility.pdf (129kB)
This file has been downloaded 966 times
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
RB - Thank you very much for those.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Our page format is falling off the ends of the earth....
To those who post pictures: PLEASE reduce your size level if you see it distorts the page format. This has implications of both "read-ability" and if
someone else happens (look how far you have to go to get to the "edit" button!)
to do that, the page can become totally illegible.
It's best not to "TILE" the pictures in any event.
Post one after another or drop them to 400/600 but
keep the sharp synthesis photo separate so everyone
can see the color.
[Edited on 5-8-2010 by quicksilver]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
nitrate reciprocal pairs, double salts, and eutectics
The use of specific combinations of nitrates in a specific proportion appears to be a valid strategy for loading up of solutions with the highest
content of total nitrates in solution for a given temperature. And it also appears that specific combinations of nitrates do form double salts and
eutectics which are capable of exluding some or all of the water of hydration which would normally have such great affinity for one or more of the
nitrates that it would otherwise be impossible to obtain that nitrate in anhydrous form by heating to drive away the water and leave the completely
dry salt. Magnesium nitrate is the nitrate of particular interest. While this is not fully described as being verified by weighing and moisture
analysis figures, some references are indicating that Magnesium Nitrate forms anhydrous double salts and/or eutectic melts with Ammonium Nitrate, with
Sodium Nitrate, with Potassium Nitrate, and other nitrates. Some combinations are of particular interest having potential value with regards to
nitration mixtures where the dehydrating property of the nitrate and / or the sulfate which may be produced as a byproduct, can improve the nitrating
power of the acid by sequestering H2O already present or H2O produced as a nitration or nitrolysis byproduct. Information in the literature is not
very extensive concerning this potentially valuable technique which could be applied not only to nitration and nitrolysis mixtures, but could also
have value in formulation of oxidizer melt mixtures for pyrotechnic compositions. There are a few mentions of this scattered among the patent
references for fertiliziers, and pyrotechnic related compositions, and for energy storage by phase change salt systems, but there is very little
information found pertaining to the usefulness for nitrations. I have found a few more references that I can share concerning the reportedly
anhydrous melts. I also found a more extensive solubility table for different temperature aqueous solutions related to the reciprocal solubility of
KNO3 and NaNO3. I will add to these mixed nitrates references as I find any more.
Attachment: Reciprocal Solubility KNO3 and NaNO3 warm solutions.pdf (132kB)
This file has been downloaded 1208 times
Attachment: Ammonium Magnesium Nitrate solubility.pdf (118kB)
This file has been downloaded 943 times
Attachment: US3729351 Eutectic Oxidizer mixtures for FLARE_COMPOSITION.pdf (103kB)
This file has been downloaded 1386 times
Attachment: GB382368 Anhydrous NaNO3 - Mg(NO3)2 - Ca(NO3)2 tertiary eutectic 138C.pdf (341kB)
This file has been downloaded 928 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Mg(NO3)2 - NH4NO3 double salts and eutectics
Here is some additional data from a Monsanto patent US3173756 and a journal article by the inventor regarding the anhydrous binary system Mg(NO3)2 -
NH4NO3 double salts and eutectic mixtures. Also attached is the US1952849 patent parallel issue to the British patent already posted above. Example
3 which is 65% anhydrous Mg(NO3)2 - 35% KNO3 is interesting. Example 4 which is 51.8% anhydrous Mg(NO3)2 - 48.2% NaNO3 is also very interesting.
I have found mention that there exists a ternary anhydrous eutectic system of slightly lower melting point for Mg(NO3)2 - KNO3 - NaNO3 but I have not
found the ratios stated.
Also it would seem that a quaternary system with those three and NH4NO3 would be likely, as well as ternary systems of Mg(NO3)2 - NH4NO3 - NaNO3 (or)
KNO3 , but I have not found any of them published. There are mentioned also systems where urea is an added component which can substantially
decrease the melting point and also result in anhydrous mixtures. Heating along with vacuum may be required for complete dehydration of some of these
mixtures at reasonably low temperatures, while vacuum may not be strictly required for others. I have no specific information what is the spread on
that, however vacuum appears to be a drying rate increaser more than an absolute requirement.
Attachment: US3173756 Griffith Magnesium Nitrate Ammonium Nitrate Anhydrous Double Salt and Inventor Journal article.pdf (884kB)
This file has been downloaded 2244 times
Attachment: US1952849 Mg(NO3)2 Anhydrous Eutectics.pdf (185kB)
This file has been downloaded 890 times
[Edited on 30-8-2010 by Rosco Bodine]
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
US PATENT 1840229, jan5,1932. (double salts)
Aimed primarily @ producing less hygroscopic,high nitrate fertilizers incorporating a wide variety of nitrates.
FWIW excuse the nonexistent link I have a hard copy of the patent
"I have now found that nitrogenous products which do not have said objections are obtained in a simple manner by combining the said basic nitrates
with the nitrates of alkali metals such as potassium,or monovalent radicles such as ammonium or urea radicals to form double salts.
[Edited on 27-1-2011 by grndpndr]
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
I try to make styphnic acid and lead styphnate like in that site powerlabs but the result was a brown "misery" not yellow, I dissolved that in a mixture of water with ethanol and heated with intention to make
more pure but all was a black colored mixture and I add some lead oxide and in the attachment picture is my disastrous result.
Can extract and purify lead styphnate or styphnic acid from my final result or is good to drop to garbage?
What was wrong with my synthesis, I follow procedure from powerlabs but I don`t have thermometers and anyway I don`t leave the mixture to become too
hot,next time I will use your procedure from first page for my 4 grams of remaining resorcinol

|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Obviously it's not possible to comment on your synthesis as you didn't post it. However whatever you did, did not have the desired result.
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
Here is my synthesis :
-8grams of powdered resorcinol are weighted and added to the 250ml beaker containing the 10ml of concentrated sulfuric acid.
-the mixture is stirred for several minutes untill the resorcinol dissolves and forms into a yellow liquid and heated for 30 minutes during which it
quickly solidifies into a pink - brown cream
- the resultant product is than chilled to -5C on a water bath with salt added, and 15ml of nitric acid are added
-the mixture react very slowly but when I heated on the hotplate reaction become more fast with NO2 fumes
-after complete reaction the mixture color become brown-black and I added 100ml distilled water
-the mixture is cooled to 5C so as to precipitate styphnic acid (or what I make) , and than was quickly filtered and washed with 400mL of cold
distilled water
-I dissolve the resultant filtrate in a boiling mixture of 1 volume ethanol and 2 volumes water and I add some amount of lead monoxide
You see the result , and that was synthesis followed by me
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Periods of time and crystal "set-up" periods were not outlined so I will assume nothing but what had been written. However the idea is that the
styphnic acid be allowed to form, crystallized and dry prior to moving onward to the synthesis of the lead salt. If that period of time and formation
is missed it's possible that is why the synthesis failed. There needs to be ordered "steps" in this process and it should not be rushed. Since I was
not looking over your shoulder; I certainly cannot say for sure but the progression has some important time elements to it.
From what I have seen the "powerlabs" synth was not a notably successful one; that's why there have been many other designs implemented. It's not too
difficult & many have had great results but the general lab to lead styphnate must progress in stages wherein the synthesis is well defined. I've
seen great success with both trinitro and lead-trinitroresorcinol labs which offered good "repeat-ability" but they had strong emphasis on time and
temperature. Very often when a nitration fails those two factors play a powerful role in the failure.
Whenever one has a problem with nitration/esterfications or where there is a sulfonation prior to the process one should always make every attempt to
look to temperature, timing, addition of precursor. & consistency. These are the major issues that result in success or failure.
[Edited on 17-4-2011 by quicksilver]
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
Quicksilver I try your synthesis on the first page and the resultant product was a yellow- red crystals, not very pure stypnic acid but my first
accomplish synthesis and I don`t have thermometer or something else to control temperature.
Tomorrow will try a recrystallization and next step will be to make lead stypnate and silver stypnate
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Unless you can control elemental conditions such as temperature, you'll never get a very pure product in many synthesis. Controls of variables such as
time, exposure, temperature, volume, & density are vital.
You can get yourself a lead salt and see if you can produce a functional lead styphnate; that would tell you something. The synthesis I use produces
a remarkably potent product & resultant lead stypnhate is of high quality, good yield & brisant (for lead styphnate anyway). The emphasis on
a careful sulfonation was suggested to another fellow's web site as his synth for the same had recurrent troubles.
[Edited on 2-5-2011 by quicksilver]
|
|
|
| Pages:
1
2
3
4 |